The presynaptic machinery at the synapse of C. elegans
- PMID: 29532181
- PMCID: PMC5851683
- DOI: 10.1007/s10158-018-0207-5
The presynaptic machinery at the synapse of C. elegans
Abstract
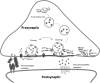
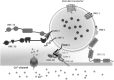
Synapses are specialized contact sites that mediate information flow between neurons and their targets. Important physical interactions across the synapse are mediated by synaptic adhesion molecules. These adhesions regulate formation of synapses during development and play a role during mature synaptic function. Importantly, genes regulating synaptogenesis and axon regeneration are conserved across the animal phyla. Genetic screens in the nematode Caenorhabditis elegans have identified a number of molecules required for synapse patterning and assembly. C. elegans is able to survive even with its neuronal function severely compromised. This is in comparison with Drosophila and mice where increased complexity makes them less tolerant to impaired function. Although this fact may reflect differences in the function of the homologous proteins in the synapses between these organisms, the most likely interpretation is that many of these components are equally important, but not absolutely essential, for synaptic transmission to support the relatively undemanding life style of laboratory maintained C. elegans. Here, we review research on the major group of synaptic proteins, involved in the presynaptic machinery in C. elegans, showing a strong conservation between higher organisms and highlight how C. elegans can be used as an informative tool for dissecting synaptic components, based on a simple nervous system organization.
Keywords: C. elegans; Synapse; Synaptic proteins; Synaptic vesicles; Synaptogenesis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Abraham C, Bai L, Leube RE. Synaptogyrin-dependent modulation of synaptic neurotransmission in Caenorhabditis elegans. Neuroscience. 2011;190:75–88. - PubMed
-
- Ann K, Kowalchyk JA, Loyet KM, Martin TF. Novel Ca2+-binding protein (CAPS) related to UNC-31 required for Ca2+-activated exocytosis. J Biol Chem. 1997;272(32):19637–19640. - PubMed
-
- Aravamudan B, Broadie K. Synaptic drosophila UNC-13 is regulated by antagonistic G-protein pathways via a proteasome-dependent degradation mechanism. J Neurobiol. 2003;54(3):417–438. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

