Comparative characterization of SHED and DPSCs during extended cultivation in vitro
- PMID: 29532869
- PMCID: PMC5928637
- DOI: 10.3892/mmr.2018.8725
Comparative characterization of SHED and DPSCs during extended cultivation in vitro
Abstract
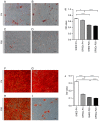
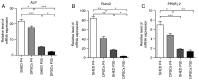
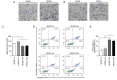
Dental pulp stem cells (DPSCs) and stem cells from human exfoliated deciduous teeth (SHED) are types of human dental tissue‑derived mesenchymal stem cells (MSCs). These cells possess a capacity for self‑renewal, multilineage differentiation potential and immunomodulatory functions. Previous studies have reported that DPSCs and SHED may be beneficial in regenerative treatments and immunotherapy. The substantial expansion of cells in vitro is a prerequisite to obtaining adequate cell numbers required for cell‑based therapy. However, the regeneration and clinical potential of MSCs diminishes with long‑term cell culture amplification. To assess the alterations in SHED and DPSCs characteristics that underlie cellular senescence and result from extended in vitro amplification, the biological properties of SHED and DPSCs at passages 4 (P4) and 20 (P20) were compared. The cells underwent senescence following serial expansion to P20, as determined by altered cell morphology, decreased proliferation and migration capacity, attenuated differentiation potential, elevated senescence‑associated β‑galactosidase (SA‑β‑gal)‑positive rates and increased apoptosis. The phenotypic changes were also accompanied by a marked increase in the expression of p53, p21 and p16Ink4a. The present study also identified that senescent DPSCs exhibited an increased number of positive cells in SA‑β‑gal staining and demonstrated varying expressions of p53, p21 and p16Ink4a in comparison with SHED, indicating the involvement of diverse pathways in cellular senescence during long‑term sequential in vitro culture and passage. Furthermore, at early and late passages, SHED exhibited a higher proliferation rate and osteogenic differentiation capability when compared with DPSCs. In addition, both cell types maintained their characteristic immunophenotype during long‑term cultivation, while the expression levels of CD73 were higher in SHED at P20. The present study concluded that notable alterations were exhibited in SHED and DPSCs during the process of extensive expansion in vitro and the results may provide guidance for the selection of safe and effective expanded SHED and DPSCs for regenerative medicine and therapy.
Keywords: dental pulp stem cells; stem cells from human exfoliated deciduous teeth; biological characteristics; cellular senescence; in vitro cultivation.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

