Tree shrew neural stem cell transplantation promotes functional recovery of tree shrews with a hemi‑sectioned spinal cord injury by upregulating nerve growth factor expression
- PMID: 29532893
- PMCID: PMC5881798
- DOI: 10.3892/ijmm.2018.3553
Tree shrew neural stem cell transplantation promotes functional recovery of tree shrews with a hemi‑sectioned spinal cord injury by upregulating nerve growth factor expression
Abstract
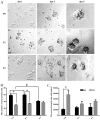
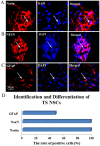
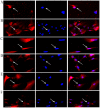
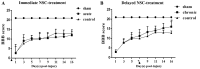
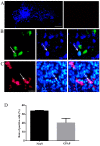
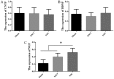
The aim of the present study was to determine the effect of implanted neural stem cells (NSCs) on the functional recovery of tree shrews (TSs) subjected to hemi‑sectioned spinal cord injury (hSCI), and to investigate the possible mechanism involved. NSCs (passage 2), derived from the hippocampus of TSs (embryonic day 20), were labeled with Hoechst 33342 and transplanted intraspinally into the hSC of TSs at thoracic level 10 in the acute (immediately after injury) and chronic (day 9 post‑injury) stages. The Basso‑Beattie‑Bresnahan (BBB) score was recorded from days 1 to 16 post‑injury, and the survival, migration, differentiation and neurotrophic factor (NTF) expression in vivo were detected. In vitro and in vivo, the expanded NSCs were able to differentiate into neurons and astrocytes, and secreted a variety of NTFs, including ciliary NTF, transforming growth factor‑β1, glial cell line‑derived NTF, nerve growth factor (NGF), brain‑derived NTF and insulin‑like growth factor. Following transplantation, the BBB score in the TSs with chronic‑stage transplantation exhibited a statistically significant increase, while there was no significant difference in the acute group, compared with the control group. This corresponded with the marked upregulation of NGF indicated by reverse transcription‑quantitative polymerase chain reaction. In conclusion, the transplantation of NSCs into the hSC in the chronic phase, but not the acute stage, of hSCI in non‑human primate TSs is effective and associated with upregulated NGF expression. These findings may provide novel strategies for the treatment of SCI in clinical patients.
Figures
Similar articles
-
Bone Marrow Mesenchymal Stem-Cell Transplantation Promotes Functional Improvement Associated with CNTF-STAT3 Activation after Hemi-Sectioned Spinal Cord Injury in Tree Shrews.Front Cell Neurosci. 2017 Jun 28;11:172. doi: 10.3389/fncel.2017.00172. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28701922 Free PMC article.
-
Transplantation of NSCs with OECs alleviates neuropathic pain associated with NGF downregulation in rats following spinal cord injury.Neurosci Lett. 2013 Aug 9;549:103-8. doi: 10.1016/j.neulet.2013.06.005. Epub 2013 Jun 20. Neurosci Lett. 2013. PMID: 23791854
-
Comparison of the properties of neural stem cells of the hippocampus in the tree shrew and rat in vitro.Mol Med Rep. 2018 Apr;17(4):5676-5683. doi: 10.3892/mmr.2018.8589. Epub 2018 Feb 12. Mol Med Rep. 2018. PMID: 29436662 Free PMC article.
-
[Neural stem cells transplantation promote the expressions of brain derived neurotrophic factor after the spinal cord injury of rats].Zhongguo Gu Shang. 2008 Nov;21(11):836-8. Zhongguo Gu Shang. 2008. PMID: 19143246 Chinese.
-
[Transplantation of neural stem cells for spinal cord injury].Rinsho Shinkeigaku. 2005 Nov;45(11):874-6. Rinsho Shinkeigaku. 2005. PMID: 16447750 Review. Japanese.
Cited by
-
Subcutaneous Maturation of Neural Stem Cell-Loaded Hydrogels Forms Region-Specific Neuroepithelium.Cells. 2018 Oct 17;7(10):173. doi: 10.3390/cells7100173. Cells. 2018. PMID: 30336590 Free PMC article.
-
Establishment of Neurobehavioral Assessment System in Tree Shrew SCT Model.J Mol Neurosci. 2020 Mar;70(3):308-319. doi: 10.1007/s12031-019-01414-9. Epub 2019 Dec 16. J Mol Neurosci. 2020. PMID: 31845102
-
From Adipose to Action: Reprogramming Stem Cells for Functional Neural Progenitors for Neural Regenerative Therapy.Int J Mol Sci. 2025 Jul 9;26(14):6599. doi: 10.3390/ijms26146599. Int J Mol Sci. 2025. PMID: 40724849 Free PMC article. Review.
-
Advances in spinal cord injury: insights from non-human primates.Neural Regen Res. 2024 Nov 1;19(11):2354-2364. doi: 10.4103/NRR.NRR-D-23-01505. Epub 2024 Jan 31. Neural Regen Res. 2024. PMID: 38526271 Free PMC article.
-
Regulating Endogenous Neural Stem Cell Activation to Promote Spinal Cord Injury Repair.Cells. 2022 Mar 1;11(5):846. doi: 10.3390/cells11050846. Cells. 2022. PMID: 35269466 Free PMC article. Review.
References
-
- Rognoni C, Fizzotti G, Pistarini C, Quaglini S. Quality of life of patients with spinal cord injury in Italy: Preliminary evaluation. Stud Health Technol Inform. 2014;205:935–939. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

