Bacterial growth, flow, and mixing shape human gut microbiota density and composition
- PMID: 29533125
- PMCID: PMC6287699
- DOI: 10.1080/19490976.2018.1448741
Bacterial growth, flow, and mixing shape human gut microbiota density and composition
Abstract
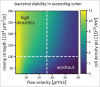
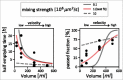
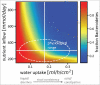
The human gut microbiota is highly dynamic, and host physiology and diet exert major influences on its composition. In our recent study, we integrated new quantitative measurements on bacterial growth physiology with a reanalysis of published data on human physiology to build a comprehensive modeling framework. This can generate predictions of how changes in different host factors influence microbiota composition. For instance, hydrodynamic forces in the colon, along with colonic water absorption that manifests as transit time, exert a major impact on microbiota density and composition. This can be mechanistically explained by their effect on colonic pH which directly affects microbiota competition for food. In this addendum, we describe the underlying analysis in more detail. In particular, we discuss the mixing dynamics of luminal content by wall contractions and its implications for bacterial growth and density, as well as the broader implications of our insights for the field of gut microbiota research.
Keywords: Bacteroidetes; Firmicutes; bacterial growth; colon physiology; colonic pH; human gut microbiota; stool consistency; water-uptake.
Figures

References
-
- Holdeman LV, Cato EP, Moore W. Anaerobic Laboratory Manual. 1977;1–140.
-
- Macfarlane GT, Gibson GR. Carbohydrate Fermentation, Energy Transduction and Gas Metabolismin the Human Large Intestine In: Mackie R, White B, Isaacson RE, editors. Gastrointestinal Microbiology. Springer Science & Business Media; 1997. p. 269–318.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
