Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury
- PMID: 29533186
- PMCID: PMC5849415
- DOI: 10.7554/eLife.32058
Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury
Abstract
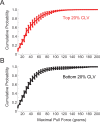
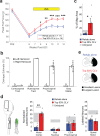
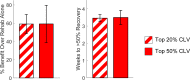
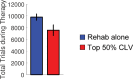
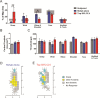
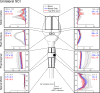
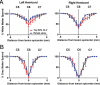
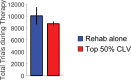
Recovery from serious neurological injury requires substantial rewiring of neural circuits. Precisely-timed electrical stimulation could be used to restore corrective feedback mechanisms and promote adaptive plasticity after neurological insult, such as spinal cord injury (SCI) or stroke. This study provides the first evidence that closed-loop vagus nerve stimulation (CLV) based on the synaptic eligibility trace leads to dramatic recovery from the most common forms of SCI. The addition of CLV to rehabilitation promoted substantially more recovery of forelimb function compared to rehabilitation alone following chronic unilateral or bilateral cervical SCI in a rat model. Triggering stimulation on the most successful movements is critical to maximize recovery. CLV enhances recovery by strengthening synaptic connectivity from remaining motor networks to the grasping muscles in the forelimb. The benefits of CLV persist long after the end of stimulation because connectivity in critical neural circuits has been restored.
Keywords: neuroscience; rat; recovery; rehabilitation; spinal cord injury; vagal nerve stimulation; vagus nerve stimulation.
© 2018, Ganzer et al.
Conflict of interest statement
PG, MD, EM, BS, AR, NR, KA, JJ, HJ, AB, MG, DP, SH, RR No competing interests declared, MK has a financial interesting in MicroTransponder, Inc., which is developing CLV for stroke and tinnitus
Figures







Comment in
-
Is the vagus nerve our neural connectome?Elife. 2018 Mar 16;7:e35592. doi: 10.7554/eLife.35592. Elife. 2018. PMID: 29546841 Free PMC article.
References
-
- Alvarez-Dieppa AC, Griffin K, Cavalier S, McIntyre CK. Vagus nerve stimulation enhances extinction of conditioned fear in rats and modulates arc protein, CaMKII, and GluN2B-Containing NMDA receptors in the basolateral amygdala. Neural Plasticity. 2016;2016:1–11. doi: 10.1155/2016/4273280. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

