Neurokinin 3 receptor antagonism rapidly improves vasomotor symptoms with sustained duration of action
- PMID: 29533369
- PMCID: PMC6092106
- DOI: 10.1097/GME.0000000000001090
Neurokinin 3 receptor antagonism rapidly improves vasomotor symptoms with sustained duration of action
Abstract
Objective: Seventy percent of postmenopausal women experience vasomotor symptoms, which can be highly disruptive and persist for years. Hormone therapy and other treatments have variable efficacy and/or side effects. Neurokinin B signaling increases in response to estrogen deficiency and has been implicated in hot flash (HF) etiology. We recently reported that a neurokinin 3 receptor (NK3R) antagonist reduces HF in postmenopausal women after 4 weeks of treatment. In this article we report novel data from that study, which shows the detailed time course of this effect.
Methods: Randomized, double-blind, placebo-controlled, single-center, crossover trial of an oral NK3R antagonist (MLE4901) for vasomotor symptoms in women aged 40 to 62 years, experiencing ≥7 HF/24 hours some of which were reported as bothersome or severe (Clinicaltrials.gov NCT02668185). Thirty-seven women were randomized and included in an intention-to-treat analysis. To ascertain the therapeutic profile of MLE4901, a post hoc time course analysis was completed.
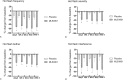
Results: By day 3 of treatment with MLE4901, HF frequency reduced by 72% (95% CI, -81.3 to -63.3%) compared with baseline (51 percentage point reduction compared with placebo, P < 0.0001); this effect size persisted throughout the 4-week dosing period. HF severity reduced by 38% compared with baseline by day 3 (95% CI, -46.1 to -29.1%) (P < 0.0001 compared with placebo), bother by 39% (95% CI, -47.5 to -30.1%) (P < 0.0001 compared with placebo), and interference by 61% (95% CI, -79.1 to -43.0%) (P = 0.0006 compared with placebo); all continued to improve throughout the 4-week dosing period (to -44%, -50%, and -70%, respectively by day 28, all P < 0.0001 compared with placebo).
Conclusions: NK3R antagonism rapidly relieves vasomotor symptoms without the need for estrogen exposure.
Conflict of interest statement
Financial disclosure/conflicts of interest: JKP is funded by the UK MRC. RER and SC are funded by the NIHR. CNJ is an inventor on a patent application 14762086.8-1453, which is registered to Imperial Innovations. PM and VHL are employees of Millendo Therapeutics. NP has lectured for and acted in an advisory capacity for Abbott, Bayer, Besins, Consilient, Meda, MSD, Mylan, Novo Nordisk, Pfizer, and Shionogi. LCW is an employee of AstraZeneca UK. AstraZeneca licensed the compound and associated patents to Millendo Therapeutics (WO2014170648A1, pending; US9475773 B2, granted). WSD is funded by the NIHR, is an inventor on a patent application 14762086.8-1453, which is registered to Imperial Innovations, and was previously an investigator for a separate study of MLE4901 in polycystic ovarian syndrome, for which a consultancy fee was paid. Millendo Therapeutics provided some financial support for an administrative assistant for the study of MLE4901 in menopausal flushing. TPS worked as a statistical consultant for Millendo Therapeutics. ANC and MSH declare no competing interests.
Figures

Comment in
-
Neurokinin 3 receptor antagonists for treatment of vasomotor symptoms: a new panacea or just a flash in the pan?Menopause. 2018 Aug;25(8):859-861. doi: 10.1097/GME.0000000000001144. Menopause. 2018. PMID: 29870472 No abstract available.
References
-
- Stearns V, Ullmer L, Lopez JF, Smith Y, Isaacs C, Hayes D. Hot flushes. Lancet 2002; 360:1851–1861. - PubMed
-
- National Institute for Health and Care Excellence. Menopause: Diagnosis and Management; 2015. Available at: https://www.nice.org.uk/guidance/ng23/chapter/recommendations Accessed August 7, 2017. - PubMed
Publication types
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

