OC-STAMP promotes osteoclast fusion for pathogenic bone resorption in periodontitis via up-regulation of permissive fusogen CD9
- PMID: 29533736
- PMCID: PMC5998976
- DOI: 10.1096/fj.201701424R
OC-STAMP promotes osteoclast fusion for pathogenic bone resorption in periodontitis via up-regulation of permissive fusogen CD9
Abstract
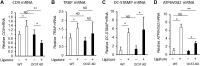
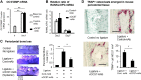
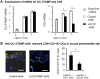
Cell fusion-mediated formation of multinuclear osteoclasts (OCs) plays a key role in bone resorption. It is reported that 2 unique OC-specific fusogens [ i.e., OC-stimulatory transmembrane protein (OC-STAMP) and dendritic cell-specific transmembrane protein (DC-STAMP)], and permissive fusogen CD9, are involved in OC fusion. In contrast to DC-STAMP-knockout (KO) mice, which show the osteopetrotic phenotype, OC-STAMP-KO mice show no difference in systemic bone mineral density. Nonetheless, according to the ligature-induced periodontitis model, significantly lower level of bone resorption was found in OC-STAMP-KO mice compared to WT mice. Anti-OC-STAMP-neutralizing mAb down-modulated in vitro: 1) the emergence of large multinuclear tartrate-resistant acid phosphatase-positive cells, 2) pit formation, and 3) mRNA and protein expression of CD9, but not DC-STAMP, in receptor activator of NF-κB ligand (RANKL)-stimulated OC precursor cells (OCps). While anti-DC-STAMP-mAb also down-regulated RANKL-induced osteoclastogenesis in vitro, it had no effect on CD9 expression. In our mouse model, systemic administration of anti-OC-STAMP-mAb suppressed the expression of CD9 mRNA, but not DC-STAMP mRNA, in periodontal tissue, along with diminished alveolar bone loss and reduced emergence of CD9+ OCps and tartrate-resistant acid phosphatase-positive multinuclear OCs. The present study demonstrated that OC-STAMP partners CD9 to promote periodontal bone destruction by up-regulation of fusion during osteoclastogenesis, suggesting that anti-OC-STAMP-mAb may lead to the development of a novel therapeutic regimen for periodontitis.-Ishii, T., Ruiz-Torruella, M., Ikeda, A., Shindo, S., Movila, A., Mawardi, H., Albassam, A., Kayal, R. A., Al-Dharrab, A. A., Egashira, K., Wisitrasameewong, W., Yamamoto, K., Mira, A. I., Sueishi, K., Han, X., Taubman, M. A., Miyamoto, T., Kawai, T. OC-STAMP promotes osteoclast fusion for pathogenic bone resorption in periodontitis via up-regulation of permissive fusogen CD9.
Keywords: DC-STAMP; RANKL; mouse model; osteoclastogenesis; periodontal bone loss.
Conflict of interest statement
This work was supported, in part, by U.S. National Institutes of Health (NIH) National Institute of Dental and Craniofacial Research Grants DE-018499, DE-019917, and T32 DE 007327-12, NIH National Institute on Aging Grant AG-053615, and a research grant from King Abdulaziz University. The authors declare no conflicts of interest.
Figures






References
-
- Xing L., Xiu Y., Boyce B. F. (2012) Osteoclast fusion and regulation by RANKL-dependent and independent factors. World J. Orthop. 3, 212–222 https://doi.org/10.5312/wjo.v3.i12.212 - DOI - PMC - PubMed
-
- Yagi M., Miyamoto T., Sawatani Y., Iwamoto K., Hosogane N., Fujita N., Morita K., Ninomiya K., Suzuki T., Miyamoto K., Oike Y., Takeya M., Toyama Y., Suda T. (2005) DC-STAMP is essential for cell–cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 202, 345–351 https://doi.org/10.1084/jem.20050645 - DOI - PMC - PubMed
-
- Witwicka H., Hwang S. Y., Reyes-Gutierrez P., Jia H., Odgren P. E., Donahue L. R., Birnbaum M. J., Odgren P. R. (2015) Studies of OC-STAMP in osteoclast fusion: a new knockout mouse model, rescue of cell fusion, and transmembrane topology. PLoS One 10, e0128275 https://doi.org/10.1371/journal.pone.0128275 - DOI - PMC - PubMed
-
- Yang M., Birnbaum M. J., MacKay C. A., Mason-Savas A., Thompson B., Odgren P. R. (2008) Osteoclast stimulatory transmembrane protein (OC-STAMP), a novel protein induced by RANKL that promotes osteoclast differentiation. J. Cell. Physiol. 215, 497–505 https://doi.org/10.1002/jcp.21331 - DOI - PMC - PubMed
-
- Miyamoto H., Suzuki T., Miyauchi Y., Iwasaki R., Kobayashi T., Sato Y., Miyamoto K., Hoshi H., Hashimoto K., Yoshida S., Hao W., Mori T., Kanagawa H., Katsuyama E., Fujie A., Morioka H., Matsumoto M., Chiba K., Takeya M., Toyama Y., Miyamoto T. (2012) Osteoclast stimulatory transmembrane protein and dendritic cell–specific transmembrane protein cooperatively modulate cell–cell fusion to form osteoclasts and foreign body giant cells. J. Bone Miner. Res. 27, 1289–1297 https://doi.org/10.1002/jbmr.1575 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

