Bcr-Abl regulation of sphingomyelin synthase 1 reveals a novel oncogenic-driven mechanism of protein up-regulation
- PMID: 29533737
- PMCID: PMC6044059
- DOI: 10.1096/fj.201701016R
Bcr-Abl regulation of sphingomyelin synthase 1 reveals a novel oncogenic-driven mechanism of protein up-regulation
Abstract
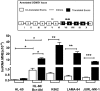
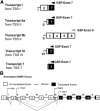
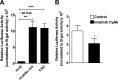
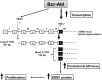
Bcr-Abl (break-point cluster region-abelson), the oncogenic trigger of chronic myelogenous leukemia (CML), has previously been shown to up-regulate the expression and activity of sphingomyelin synthase 1 (SMS1), which contributes to the proliferation of CML cells; however, the mechanism by which this increased expression of SMS1 is mediated remains unknown. In the current study, we show that Bcr-Abl enhances the expression of SMS1 via a 30-fold up-regulation of its transcription. Of most interest, the Bcr-Abl-regulated transcription of SMS1 is initiated from a novel transcription start site (TSS) that is just upstream of the open reading frame. This shift in TSS utilization generates an SMS1 mRNA with a substantially shorter 5' UTR compared with its canonical mRNA. This shorter 5' UTR imparts a 20-fold greater translational efficiency to SMS1 mRNA, which further contributes to the increase of its expression in CML cells. Therefore, our study demonstrates that Bcr-Abl increases SMS1 protein levels via 2 concerted mechanisms: up-regulation of transcription and enhanced translation as a result of the shift in TSS utilization. Remarkably, this is the first time that an oncogene-Bcr-Abl-has been demonstrated to drive such a mechanism that up-regulates the expression of a functionally important target gene, SMS1.-Moorthi, S., Burns, T. A., Yu, G.-Q., Luberto, C. Bcr-Abl regulation of sphingomyelin synthase 1 reveals a novel oncogenic-driven mechanism of protein up-regulation.
Keywords: alternative TSS; cancer; transcription; translation; translation efficiency.
Conflict of interest statement
The authors thank Dr. Paola Signorelli (Department of Health Sciences, University of Milan, Milan, Italy) and Dr. Daniella Ishimaru (Medical University of South Carolina) for expert advice. The authors also thank Dr. Can Senkal and Dr. Yusuf Hannun (both of the Department of Medicine and Cancer Center at Stony Brook University) for critical input when writing the manuscript. The authors thank the Stony Brook DNA Sequencing facility for timely assistance. This work was supported by U.S. National Institutes of Health, National Cancer Institute Grant P01-CA097132 (to C. L. for Project #4) and the Stony Brook Scholars in Biomedical Sciences Award (to S. M.). The authors declare no conflicts of interest.
Figures




References
-
- Yamaoka S., Miyaji M., Kitano T., Umehara H., Okazaki T. (2004) Expression cloning of a human cDNA restoring sphingomyelin synthesis and cell growth in sphingomyelin synthase-defective lymphoid cells. J. Biol. Chem. 279, 18688–18693 - PubMed
-
- Bernert J. T., Jr., Ullman M. D. (1981) Biosynthesis of sphingomyelin from erythro-ceramides and phosphatidylcholine by a microsomal cholinephosphotransferase. Biochim. Biophys. Acta 666, 99–109 - PubMed
-
- Hatch G. M., Vance D. E. (1992) Stimulation of sphingomyelin biosynthesis by brefeldin A and sphingomyelin breakdown by okadaic acid treatment of rat hepatocytes. J. Biol. Chem. 267, 12443–12451 - PubMed
-
- Marggraf W. D., Anderer F. A., Kanfer J. N. (1981) The formation of sphingomyelin from phosphatidylcholine in plasma membrane preparations from mouse fibroblasts. Biochim. Biophys. Acta 664, 61–73 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

