β-Actin shows limited mobility and is required only for supraphysiological insulin-stimulated glucose transport in young adult soleus muscle
- PMID: 29533739
- PMCID: PMC6087721
- DOI: 10.1152/ajpendo.00392.2017
β-Actin shows limited mobility and is required only for supraphysiological insulin-stimulated glucose transport in young adult soleus muscle
Abstract
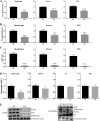
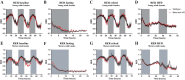
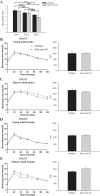
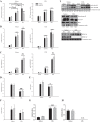
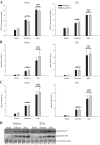
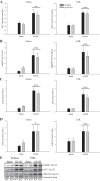
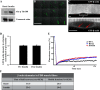
Studies in skeletal muscle cell cultures suggest that the cortical actin cytoskeleton is a major requirement for insulin-stimulated glucose transport, implicating the β-actin isoform, which in many cell types is the main actin isoform. However, it is not clear that β-actin plays such a role in mature skeletal muscle. Neither dependency of glucose transport on β-actin nor actin reorganization upon glucose transport have been tested in mature muscle. To investigate the role of β-actin in fully differentiated muscle, we performed a detailed characterization of wild type and muscle-specific β-actin knockout (KO) mice. The effects of the β-actin KO were subtle; however, we confirmed the previously reported decline in running performance of β-actin KO mice compared with wild type during repeated maximal running tests. We also found insulin-stimulated glucose transport into incubated muscles reduced in soleus but not in extensor digitorum longus muscle of young adult mice. Contraction-stimulated glucose transport trended toward the same pattern, but the glucose transport phenotype disappeared in soleus muscles from mature adult mice. No genotype-related differences were found in body composition or glucose tolerance or by indirect calorimetry measurements. To evaluate β-actin mobility in mature muscle, we electroporated green fluorescent protein (GFP)-β-actin into flexor digitorum brevis muscle fibers and measured fluorescence recovery after photobleaching. GFP-β-actin showed limited unstimulated mobility and no changes after insulin stimulation. In conclusion, β-actin is not required for glucose transport regulation in mature mouse muscle under the majority of the tested conditions. Thus, our work reveals fundamental differences in the role of the cortical β-actin cytoskeleton in mature muscle compared with cell culture.
Keywords: actin cytoskeleton; glucose transport; insulin; skeletal muscle; β-actin.
Figures


Similar articles
-
Chronic treatment with 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside increases insulin-stimulated glucose uptake and GLUT4 translocation in rat skeletal muscles in a fiber type-specific manner.Diabetes. 2001 Jan;50(1):12-7. doi: 10.2337/diabetes.50.1.12. Diabetes. 2001. PMID: 11147776
-
The Rab-GTPase-activating protein TBC1D1 regulates skeletal muscle glucose metabolism.Am J Physiol Endocrinol Metab. 2012 Aug 15;303(4):E524-33. doi: 10.1152/ajpendo.00605.2011. Epub 2012 Jun 12. Am J Physiol Endocrinol Metab. 2012. PMID: 22693207
-
Oligomeric resistin impairs insulin and AICAR-stimulated glucose uptake in mouse skeletal muscle by inhibiting GLUT4 translocation.Am J Physiol Endocrinol Metab. 2009 Jul;297(1):E57-66. doi: 10.1152/ajpendo.90744.2008. Epub 2009 May 12. Am J Physiol Endocrinol Metab. 2009. PMID: 19435854
-
Exercise-induced AMPK activity in skeletal muscle: role in glucose uptake and insulin sensitivity.Mol Cell Endocrinol. 2013 Feb 25;366(2):204-14. doi: 10.1016/j.mce.2012.06.013. Epub 2012 Jul 11. Mol Cell Endocrinol. 2013. PMID: 22796442 Review.
-
Regulation of human muscle contraction at the cellular and molecular levels.Ital J Neurol Sci. 1999 Dec;20(6):413-22. doi: 10.1007/s100720050061. Ital J Neurol Sci. 1999. PMID: 10937862 Review.
Cited by
-
p300 or CBP is required for insulin-stimulated glucose uptake in skeletal muscle and adipocytes.JCI Insight. 2022 Jan 11;7(1):e141344. doi: 10.1172/jci.insight.141344. JCI Insight. 2022. PMID: 34813504 Free PMC article.
-
Loss of β-Cytoplasmic Actin in the Intestinal Epithelium Increases Gut Barrier Permeability in vivo and Exaggerates the Severity of Experimental Colitis.Front Cell Dev Biol. 2020 Oct 23;8:588836. doi: 10.3389/fcell.2020.588836. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33195251 Free PMC article.
-
Muscle-specific AXIN1 and AXIN2 double knockout does not alter AMPK/mTORC1 signalling or glucose metabolism.J Physiol. 2025 Jul;603(14):3961-3971. doi: 10.1113/JP288854. Epub 2025 Jun 30. J Physiol. 2025. PMID: 40587294 Free PMC article.
-
Gene deletion of γ-actin impairs insulin-stimulated skeletal muscle glucose uptake in growing mice but not in mature adult mice.Physiol Rep. 2022 Feb;10(4):e15183. doi: 10.14814/phy2.15183. Physiol Rep. 2022. PMID: 35224890 Free PMC article.
-
Rho GTPases-Emerging Regulators of Glucose Homeostasis and Metabolic Health.Cells. 2019 May 9;8(5):434. doi: 10.3390/cells8050434. Cells. 2019. PMID: 31075957 Free PMC article. Review.
References
-
- Balteau M, Tajeddine N, de Meester C, Ginion A, Des Rosiers C, Brady NR, Sommereyns C, Horman S, Vanoverschelde JL, Gailly P, Hue L, Bertrand L, Beauloye C. NADPH oxidase activation by hyperglycaemia in cardiomyocytes is independent of glucose metabolism but requires SGLT1. Cardiovasc Res 92: 237–246, 2011. doi:10.1093/cvr/cvr230. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

