Unique Metabolic Adaptations Dictate Distal Organ-Specific Metastatic Colonization
- PMID: 29533780
- PMCID: PMC5889305
- DOI: 10.1016/j.ccell.2018.02.001
Unique Metabolic Adaptations Dictate Distal Organ-Specific Metastatic Colonization
Abstract
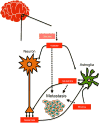
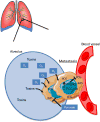
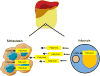
Metastases arising from tumors have the proclivity to colonize specific organs, suggesting that they must rewire their biology to meet the demands of the organ colonized, thus altering their primary properties. Each metastatic site presents distinct metabolic challenges to a colonizing cancer cell, ranging from fuel and oxygen availability to oxidative stress. Here, we discuss the organ-specific metabolic adaptations that cancer cells must undergo, which provide the ability to overcome the unique barriers to colonization in foreign tissues and establish the metastatic tissue tropism phenotype.
Keywords: cancer; metabolism; metastasis; tissue tropism.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures



References
-
- Albrecht J, Sidoryk-Wegrzynowicz M, Zielinska M, Aschner M. Roles of glutamine in neurotransmission. Neuron Glia Biol. 2010;6:263–276. - PubMed
-
- Andrzejewski S, Klimcakova E, Johnson RM, Tabaries S, Annis MG, McGuirk S, Northey JJ, Chenard V, Sriram U, Papadopoli DJ, et al. PGC-1alpha Promotes Breast Cancer Metastasis and Confers Bioenergetic Flexibility against Metabolic Drugs. Cell Metab. 2017;26:778–787 e775. - PubMed
-
- Carlinfante G, Vassiliou D, Svensson O, Wendel M, Heinegard D, Andersson G. Differential expression of osteopontin and bone sialoprotein in bone metastasis of breast and prostate carcinoma. Clin Exp Metastasis. 2003;20:437–444. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

