Vinylation of a Secondary Amine Core with Calcium Carbide for Efficient Post-Modification and Access to Polymeric Materials
- PMID: 29534039
- PMCID: PMC6017323
- DOI: 10.3390/molecules23030648
Vinylation of a Secondary Amine Core with Calcium Carbide for Efficient Post-Modification and Access to Polymeric Materials
Abstract
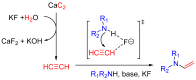
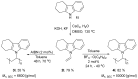
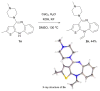
We developed a simple and efficient strategy to access N-vinyl secondary amines of various naturally occurring materials using readily available solid acetylene reagents (calcium carbide, KF, and KOH). Pyrrole, pyrazole, indoles, carbazoles, and diarylamines were successfully vinylated in good yields. Cross-linked and linear polymers were synthesized from N-vinyl carbazoles through free radical and cationic polymerization. Post-modification of olanzapine (an antipsychotic drug substance) was successfully performed.
Keywords: acetylene; calcium carbide; functionalization; olanzapine; vinylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Vinylation of Aryl Ether (Lignin β-O-4 Linkage) and Epoxides with Calcium Carbide through C-O Bond Cleavage.ChemSusChem. 2017 Aug 24;10(16):3198-3201. doi: 10.1002/cssc.201701153. Epub 2017 Aug 4. ChemSusChem. 2017. PMID: 28730737
-
A Green and Sustainable Route to Carbohydrate Vinyl Ethers for Accessing Bioinspired Materials with a Unique Microspherical Morphology.ChemSusChem. 2018 Jan 10;11(1):292-298. doi: 10.1002/cssc.201701489. Epub 2017 Dec 18. ChemSusChem. 2018. PMID: 28898575
-
TBAF-mediated reactions of 1,1-dibromo-1-alkenes with thiols and amines and regioselective synthesis of 1,2-heterodisubstituted alkenes.J Org Chem. 2011 Apr 15;76(8):2448-58. doi: 10.1021/jo2000176. Epub 2011 Mar 16. J Org Chem. 2011. PMID: 21410271
-
Hyperbranched Polymers by Light-Induced Self-Condensing Vinyl Polymerization.Macromol Rapid Commun. 2018 Aug;39(15):e1800276. doi: 10.1002/marc.201800276. Epub 2018 Jun 5. Macromol Rapid Commun. 2018. PMID: 29870586 Review.
-
A general concept for the preparation of hierarchically structured pi-conjugated polymers.Chemistry. 2008;14(10):2942-55. doi: 10.1002/chem.200701325. Chemistry. 2008. PMID: 18228550 Review.
Cited by
-
Acetylene in Organic Synthesis: Recent Progress and New Uses.Molecules. 2018 Sep 24;23(10):2442. doi: 10.3390/molecules23102442. Molecules. 2018. PMID: 30250005 Free PMC article. Review.
-
Vinylation of N-Heteroarenes through Addition/Elimination Reactions of Vinyl Selenones.Molecules. 2023 Aug 12;28(16):6026. doi: 10.3390/molecules28166026. Molecules. 2023. PMID: 37630278 Free PMC article.
-
Thermal Mapping of Self-Promoted Calcium Carbide Reactions for Performing Energy-Economic Processes.Int J Mol Sci. 2022 Mar 2;23(5):2763. doi: 10.3390/ijms23052763. Int J Mol Sci. 2022. PMID: 35269903 Free PMC article.
-
Towards Sustainable Carbon Return from Waste to Industry via C2-Type Molecular Unit.Int J Mol Sci. 2022 Oct 5;23(19):11828. doi: 10.3390/ijms231911828. Int J Mol Sci. 2022. PMID: 36233131 Free PMC article.
References
-
- Oshiro Y., Shirota Y., Mikawa H. Synthesis of polymers with polar side groups. III. Tricyanovinylation of poly(N-vinylindole) and N-Vinylindole—Fumaronitrile copolymer, and dielectric properties of these polymers. Polym. J. 1974;6:364–369. doi: 10.1295/polymj.6.364. - DOI
-
- Grazulevicius J.V., Strohriegl P., Pielichowski J., Pielichowski K. Carbazole-containing polymers: Synthesis, properties and applications. Prog. Polym. Sci. 2003;28:1297–1353. doi: 10.1016/S0079-6700(03)00036-4. - DOI
-
- Hoegl H. On photoelectric effects in polymers and their sensitization by dopants. J. Phys. Chem. 1965;69:755–766. doi: 10.1021/j100887a008. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

