Recent Advances in Zirconium-89 Chelator Development
- PMID: 29534538
- PMCID: PMC6017441
- DOI: 10.3390/molecules23030638
Recent Advances in Zirconium-89 Chelator Development
Abstract
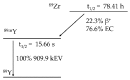
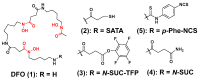
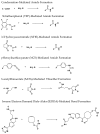
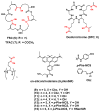
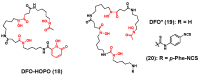
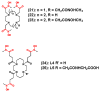
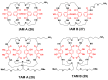
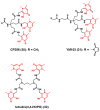
The interest in zirconium-89 (89Zr) as a positron-emitting radionuclide has grown considerably over the last decade due to its standardized production, long half-life of 78.2 h, favorable decay characteristics for positron emission tomography (PET) imaging and its successful use in a variety of clinical and preclinical applications. However, to be utilized effectively in PET applications it must be stably bound to a targeting ligand, and the most successfully used 89Zr chelator is desferrioxamine B (DFO), which is commercially available as the iron chelator Desferal®. Despite the prevalence of DFO in 89Zr-immuno-PET applications, the development of new ligands for this radiometal is an active area of research. This review focuses on recent advances in zirconium-89 chelation chemistry and will highlight the rapidly expanding ligand classes that are under investigation as DFO alternatives.
Keywords: chelator; positron emission tomography; zirconium-89.
Conflict of interest statement
N.B.B., D.N.P. and T.J.W. have filed patents relating to work described in this text.
Figures



References
-
- Ossenkoppele R., Prins N.D., Pijnenburg Y.A., Lemstra A.W., van der Flier W.M., Adriaanse S.F., Windhorst A.D., Handels R.L., Wolfs C.A., Aalten P., et al. Impact of molecular imaging on the diagnostic process in a memory clinic. Alzheimers Dement. 2013;9:414–421. doi: 10.1016/j.jalz.2012.07.003. - DOI - PubMed
-
- Hruska C.B., Boughey J.C., Phillips S.W., Rhodes D.J., Wahner-Roedler D.L., Whaley D.H., Degnim A.C., O‘Connor M.K. Scientific Impact Recognition Award: Molecular breast imaging: A review of the Mayo Clinic experience. Am. J. Surg. 2008;196:470–476. doi: 10.1016/j.amjsurg.2008.06.005. - DOI - PMC - PubMed
-
- Kipper M.S. Steps in moving molecular imaging to the clinic. J. Nucl. Med. 2008;49:58N–60N. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

