The SBRT database initiative of the German Society for Radiation Oncology (DEGRO): patterns of care and outcome analysis of stereotactic body radiotherapy (SBRT) for liver oligometastases in 474 patients with 623 metastases
- PMID: 29534687
- PMCID: PMC5851117
- DOI: 10.1186/s12885-018-4191-2
The SBRT database initiative of the German Society for Radiation Oncology (DEGRO): patterns of care and outcome analysis of stereotactic body radiotherapy (SBRT) for liver oligometastases in 474 patients with 623 metastases
Abstract
Background: The intent of this pooled analysis as part of the German society for radiation oncology (DEGRO) stereotactic body radiotherapy (SBRT) initiative was to analyze the patterns of care of SBRT for liver oligometastases and to derive factors influencing treated metastases control and overall survival in a large patient cohort.
Methods: From 17 German and Swiss centers, data on all patients treated for liver oligometastases with SBRT since its introduction in 1997 has been collected and entered into a centralized database. In addition to patient and tumor characteristics, data on immobilization, image guidance and motion management as well as dose prescription and fractionation has been gathered. Besides dose response and survival statistics, time trends of the aforementioned variables have been investigated.
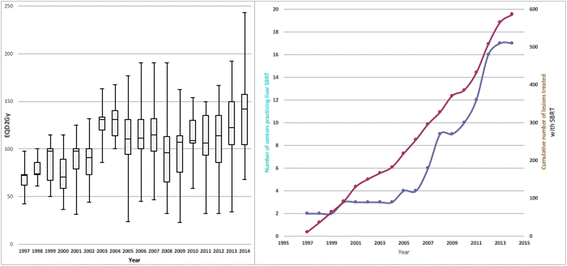
Results: In total, 474 patients with 623 liver oligometastases (median 1 lesion/patient; range 1–4) have been collected from 1997 until 2015. Predominant histologies were colorectal cancer (n = 213 pts.; 300 lesions) and breast cancer (n = 57; 81 lesions). All centers employed an SBRT specific setup. Initially, stereotactic coordinates and CT simulation were used for treatment set-up (55%), but eventually were replaced by CBCT guidance (28%) or more recently robotic tracking (17%). High variance in fraction (fx) number (median 1 fx; range 1–13) and dose per fraction (median: 18.5 Gy; range 3–37.5 Gy) was observed, although median BED remained consistently high after an initial learning curve. Median follow-up time was 15 months; median overall survival after SBRT was 24 months. One- and 2-year treated metastases control rate of treated lesions was 77% and 64%; if maximum isocenter biological equivalent dose (BED) was greater than 150 Gy EQD2Gy, it increased to 83% and 70%, respectively. Besides radiation dose colorectal and breast histology and motion management methods were associated with improved treated metastases control.
Conclusion: After an initial learning curve with regards to total cumulative doses, consistently high biologically effective doses have been employed translating into high local tumor control at 1 and 2 years. The true impact of histology and motion management method on treated metastases control deserve deeper analysis. Overall survival is mainly influenced by histology and metastatic tumor burden.
Background: The intent of this pooled analysis as part of the German society for radiation oncology (DEGRO) stereotactic body radiotherapy (SBRT) initiative was to analyze the patterns of care of SBRT for liver oligometastases and to derive factors influencing treated metastases control and overall survival in a large patient cohort.
Methods: From 17 German and Swiss centers, data on all patients treated for liver oligometastases with SBRT since its introduction in 1997 has been collected and entered into a centralized database. In addition to patient and tumor characteristics, data on immobilization, image guidance and motion management as well as dose prescription and fractionation has been gathered. Besides dose response and survival statistics, time trends of the aforementioned variables have been investigated.
Results: In total, 474 patients with 623 liver oligometastases (median 1 lesion/patient; range 1–4) have been collected from 1997 until 2015. Predominant histologies were colorectal cancer (n = 213 pts.; 300 lesions) and breast cancer (n = 57; 81 lesions). All centers employed an SBRT specific setup. Initially, stereotactic coordinates and CT simulation were used for treatment set-up (55%), but eventually were replaced by CBCT guidance (28%) or more recently robotic tracking (17%). High variance in fraction (fx) number (median 1 fx; range 1–13) and dose per fraction (median: 18.5 Gy; range 3–37.5 Gy) was observed, although median BED remained consistently high after an initial learning curve. Median follow-up time was 15 months; median overall survival after SBRT was 24 months. One- and 2-year treated metastases control rate of treated lesions was 77% and 64%; if maximum isocenter biological equivalent dose (BED) was greater than 150 Gy EQD2Gy, it increased to 83% and 70%, respectively. Besides radiation dose colorectal and breast histology and motion management methods were associated with improved treated metastases control.
Conclusion: After an initial learning curve with regards to total cumulative doses, consistently high biologically effective doses have been employed translating into high local tumor control at 1 and 2 years. The true impact of histology and motion management method on treated metastases control deserve deeper analysis. Overall survival is mainly influenced by histology and metastatic tumor burden.
Keywords: Liver oligometastases; Oligo-recurrence; Oligometastases; Outcome; Stereotactic body radiotherapy; Sync-oligometastases; Treated metastases control.
Conflict of interest statement
Authors’ information
Not applicable.
Ethics approval and consent to participate
The multicenter data collection and analysis was approved by the Ethics committee of the Kanton Zurich, Switzerland (BASEC-Nr. 2016–00744) and in addition to local regulations also covered the following institutions:
University Hospital Zürich, Department of Radiation Oncology, University of Zurich, Zurich, Switzerland.
Strahlentherapie Bautzen, Department of Radiation Oncology, Bautzen, Germany
University of Munich – LMU Munich, Department of Radiation Oncology,Munich, German
University Hospital Basel, Department of Radiation Oncology, Basel, Switzerland
University Medical Center Hamburg-Eppendorf, Department of Radiation Oncology, Hamburg, Germany
Strahlenzentrum Hamburg, Department of Radiation Oncology, Hamburg, Germany
University Hospital of Cologne, Department of Radiation Oncology, Cologne, Germany
University Hospital Würzburg, Department of Radiation Oncology, Würzburg, Germany
University Hospital Halle, Department of Radiation Oncology, Halle, Germany
Klinikum Passau, Radiation Oncology, Passau, Germany
If necessary, the data collection of the individual participating centers was approved according to local regulations and approved by the respective local ethics committees. The following ethics committees and regulatory bodies were involved in this local approval process:
Medizinische Ethik-Komission II, Medizinische Fakultät Mannheim; 2014-413 M-MA-§23bMPG: University Hospital Mannheim, Department of Radiation Oncology, University of Heidelberg, Mannheim, Germany.
Ethikkommission der Medizinischen Fakultät Heidelberg; S459–2010:
Ethikkommission der Medizinischen Fakultät der Technischen Universität München; 84/16S: Klinikum rechts der Isar- Technische Universität München, Department of Radiation Oncology, Munich, Germany
Ethikkommission an der Medizinischen Fakultät der Universität Rostock, A2016–0008:
Universitätsklinikum Schleswig-Holstein, Department of Radiation Oncology, Kiel/Lübeck, Germany.
University Hospital Rostock, Department of Radiation Oncology, Rostock, Germany.
Ethikkommission der Universität Freiburg, 462/12: University Hospital Freiburg, Department of Radiation Oncology, Freiburg, Germany
Ärztekammer: Bezirksärztekammer Nord-Württemberg, Jahnstr. 5, 70,597 Stuttgart: RadioChirurgicum CyberKnife Südwest, Radiation Oncology, Göppingen, Germany.
Ethikkommission der Bayerischen Ärztekammer, mb BO 16002: Krankenhaus Barmherzige Brüder, Department of Radiation Oncology, Regensburg, Germany
The participants consent was written as part of the main ethics approval.
Consent for publication
Not applicable.
Competing interests
Marciana Duma is a member of the editorial board (Associate editor) of BMC Cancer. NA confirms that all other authors have nothing to declare at the time of submission and that there are no competing interests to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Niibe Y, Yamashita H, Sekiguchi K, Takahashi W, Shiraishi K, Okuma K, Terahara A, Kawamori J, Nakagawa K. Stereotactic body radiotherapy results for pulmonary Oligometastases: a two-institution collaborative investigation. Anticancer Res. 2015;35:4903–4908. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

