Formation of chimeric genes with essential functions at the origin of eukaryotes
- PMID: 29534719
- PMCID: PMC5851275
- DOI: 10.1186/s12915-018-0500-0
Formation of chimeric genes with essential functions at the origin of eukaryotes
Abstract
Background: Eukaryotes evolved from the symbiotic association of at least two prokaryotic partners, and a good deal is known about the timings, mechanisms, and dynamics of these evolutionary steps. Recently, it was shown that a new class of nuclear genes, symbiogenetic genes (S-genes), was formed concomitant with endosymbiosis and the subsequent evolution of eukaryotic photosynthetic lineages. Understanding their origins and contributions to eukaryogenesis would provide insights into the ways in which cellular complexity has evolved.
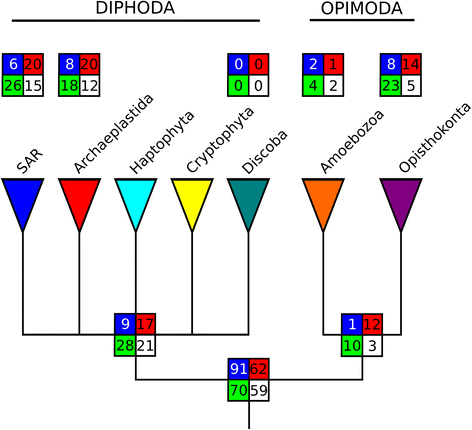
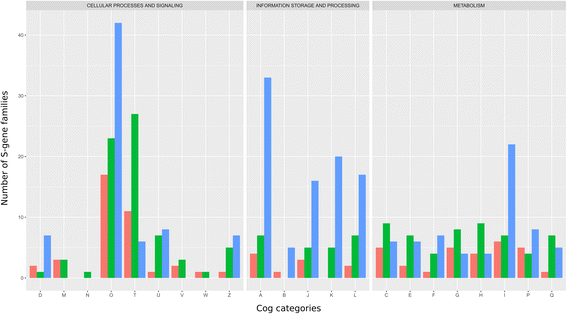
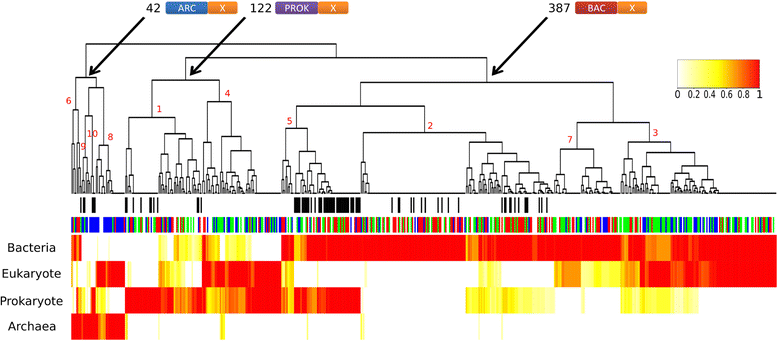
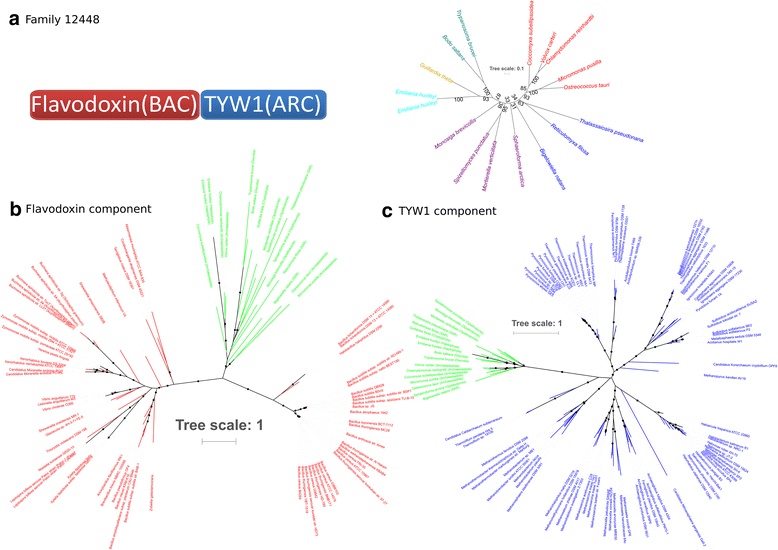
Results: Here, we show that chimeric nuclear genes (S-genes), built from prokaryotic domains, are critical for explaining the leap forward in cellular complexity achieved during eukaryogenesis. A total of 282 S-gene families contributed solutions to many of the challenges faced by early eukaryotes, including enhancing the informational machinery, processing spliceosomal introns, tackling genotoxicity within the cell, and ensuring functional protein interactions in a larger, more compartmentalized cell. For hundreds of S-genes, we confirmed the origins of their components (bacterial, archaeal, or generally prokaryotic) by maximum likelihood phylogenies. Remarkably, Bacteria contributed nine-fold more S-genes than Archaea, including a two-fold greater contribution to informational functions. Therefore, there is an additional, large bacterial contribution to the evolution of eukaryotes, implying that fundamental eukaryotic properties do not strictly follow the traditional informational/operational divide for archaeal/bacterial contributions to eukaryogenesis.
Conclusion: This study demonstrates the extent and process through which prokaryotic fragments from bacterial and archaeal genes inherited during eukaryogenesis underly the creation of novel chimeric genes with important functions.
Keywords: Chimeric genes; Endosymbiosis; Eukaryogenesis; Evolutionary genomics; Evolutionary transition.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

