RPE phagocytic function declines in age-related macular degeneration and is rescued by human umbilical tissue derived cells
- PMID: 29534722
- PMCID: PMC5851074
- DOI: 10.1186/s12967-018-1434-6
RPE phagocytic function declines in age-related macular degeneration and is rescued by human umbilical tissue derived cells
Abstract
Background: Age-related macular degeneration (AMD) is a leading cause of blindness among the elderly characterized by retinal pigment epithelium (RPE) degeneration with accumulation of abnormal intracellular deposits (lipofuscin) and photoreceptor death. RPE is vital for the retina and integrity of photoreceptors through its phagocytic function which is closely linked to formation of lipofuscin through daily phagocytosis of discarded photoreceptor outer segments (POS). Although phagocytosis has been implicated in AMD, it has not been directly shown to be altered in AMD. RPE phagocytic defect was previously shown to be rescued by subretinal injection of human umbilical tissue derived cells (hUTC) in a rodent model of retinal degeneration (RCS rat) through receptor tyrosine kinase (RTK) ligands and bridge molecules. Here, we examined RPE phagocytic function directly in the RPE from AMD patients and the ability and mechanisms of hUTC to affect phagocytosis in the human RPE.
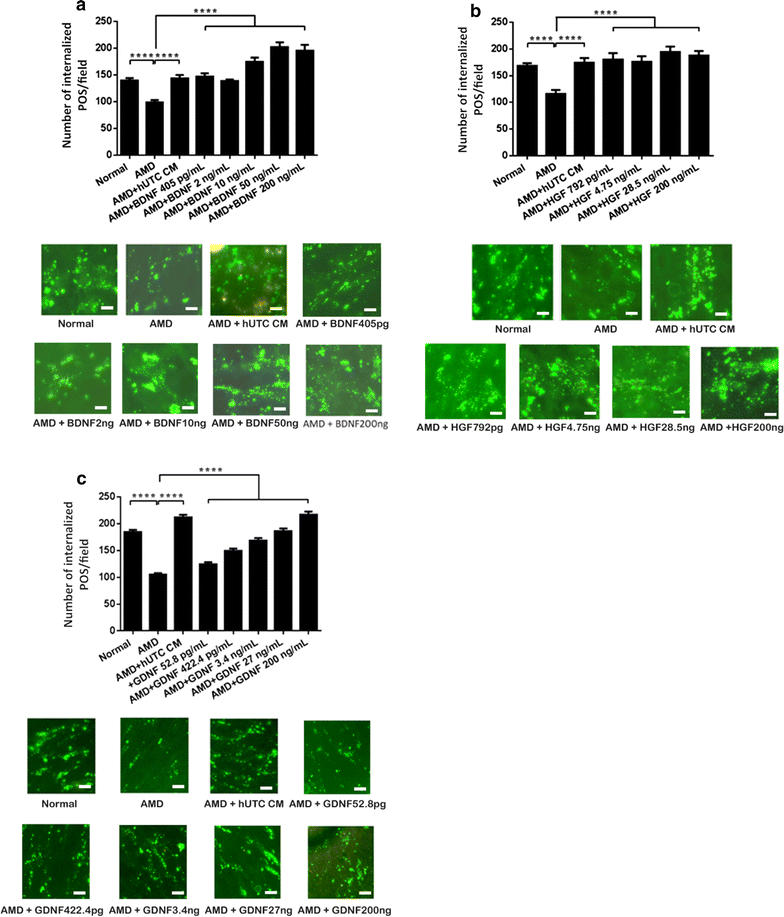
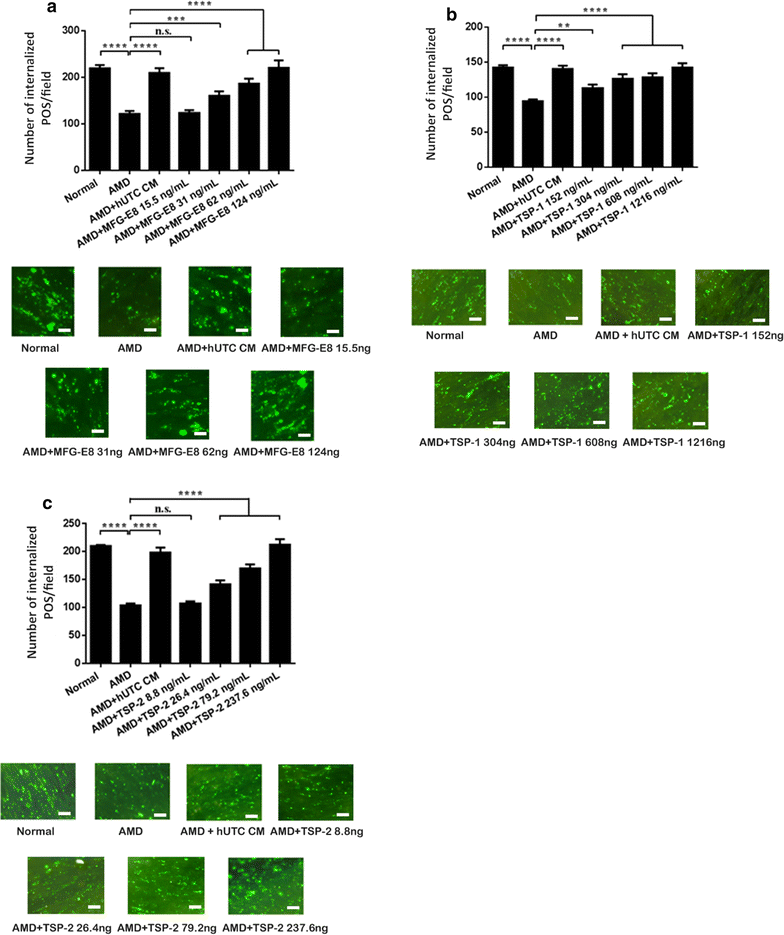
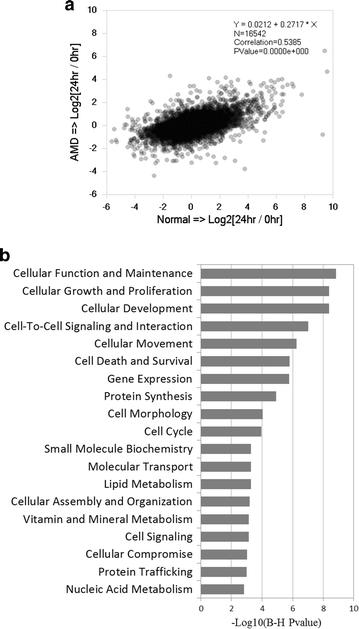
Methods: Human RPE was isolated from the post-mortem eyes of normal and AMD-affected subjects and cultured. RPE phagocytic function was measured in vitro using isolated POS. The effects of hUTC conditioned media, recombinant RTK ligands brain-derived neurotrophic factor (BDNF), hepatocyte growth factor (HGF), and glial cell-derived neurotrophic factor (GDNF), as well as bridge molecules milk-fat-globule-EGF-factor 8 (MFG-E8), thrombospondin (TSP)-1, and TSP-2 on phagocytosis were also examined in phagocytosis assays using isolated POS. RNA was isolated from normal and AMD RPE treated with hUTC conditioned media and subjected to transcriptome profiling by RNA-Seq and computational analyses.
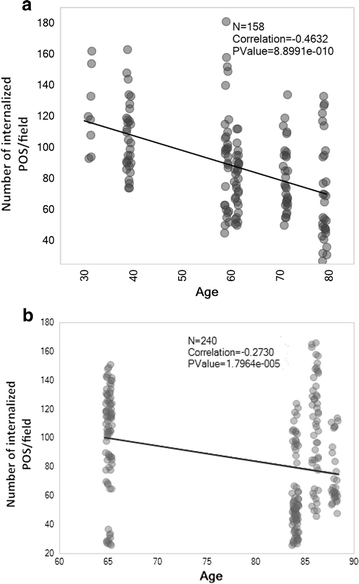
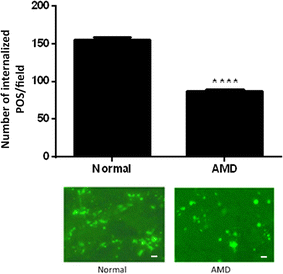
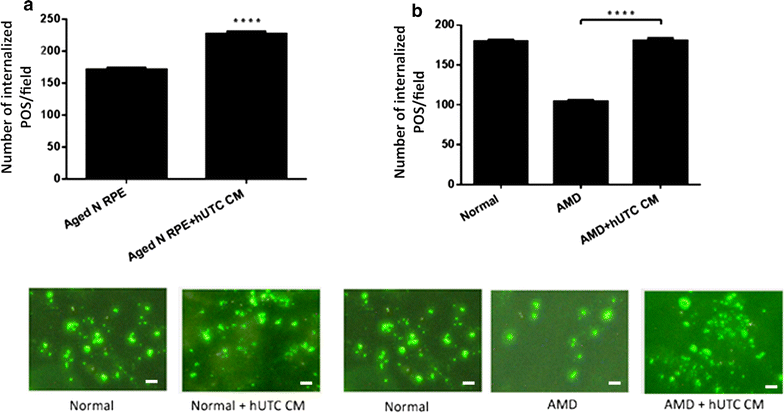
Results: RPE phagocytosis, while showing a moderate decline with age, was significantly reduced in AMD RPE, more than expected for age. hUTC conditioned media stimulated phagocytosis in the normal human RPE and significantly rescued the phagocytic dysfunction in the AMD RPE. RTK ligands and bridge molecules duplicated the rescue effect. Moreover, multiple molecular pathways involving phagocytosis, apoptosis, oxidative stress, inflammation, immune activation, and cholesterol transport were affected by hUTC in the RPE.
Conclusions: We demonstrated for the first time RPE phagocytic dysfunction in AMD, highlighting its likely importance in AMD, and the ability of hUTC to correct this dysfunction, providing insights into the therapeutic potential of hUTC for AMD.
Keywords: Age-related macular degeneration; Bridge molecules; Cell therapy; Human umbilical tissue derived cells; Phagocytosis; Receptor tyrosine kinase; Retinal pigment epithelium.
Figures
Similar articles
-
Human umbilical tissue-derived cells rescue retinal pigment epithelium dysfunction in retinal degeneration.Stem Cells. 2016 Feb;34(2):367-79. doi: 10.1002/stem.2239. Epub 2015 Nov 26. Stem Cells. 2016. PMID: 26523756
-
Characterization of the effects of retinal pigment epithelium-conditioned media on porcine and aged human retina.Graefes Arch Clin Exp Ophthalmol. 2013 Jun;251(6):1515-28. doi: 10.1007/s00417-013-2326-3. Epub 2013 Apr 11. Graefes Arch Clin Exp Ophthalmol. 2013. PMID: 23575949
-
miR-184 regulates ezrin, LAMP-1 expression, affects phagocytosis in human retinal pigment epithelium and is downregulated in age-related macular degeneration.FEBS J. 2014 Dec;281(23):5251-64. doi: 10.1111/febs.13066. Epub 2014 Oct 13. FEBS J. 2014. PMID: 25251993
-
Retinal pigment epithelium in the pathogenesis of age-related macular degeneration and photobiomodulation as a potential therapy?Clin Exp Ophthalmol. 2018 Aug;46(6):670-686. doi: 10.1111/ceo.13121. Epub 2018 Jan 12. Clin Exp Ophthalmol. 2018. PMID: 29205705 Review.
-
Stem cell based therapies for age-related macular degeneration: The promises and the challenges.Prog Retin Eye Res. 2015 Sep;48:1-39. doi: 10.1016/j.preteyeres.2015.06.004. Epub 2015 Jun 23. Prog Retin Eye Res. 2015. PMID: 26113213 Review.
Cited by
-
Exploring the pathogenesis of age-related macular degeneration: A review of the interplay between retinal pigment epithelium dysfunction and the innate immune system.Front Neurosci. 2022 Nov 3;16:1009599. doi: 10.3389/fnins.2022.1009599. eCollection 2022. Front Neurosci. 2022. PMID: 36408381 Free PMC article. Review.
-
Role of Mitochondria in Retinal Pigment Epithelial Aging and Degeneration.Front Aging. 2022 Jul 15;3:926627. doi: 10.3389/fragi.2022.926627. eCollection 2022. Front Aging. 2022. PMID: 35912040 Free PMC article. Review.
-
Location-Specific Thickness Patterns in Intermediate Age-Related Macular Degeneration Reveals Anatomical Differences in Multiple Retinal Layers.Invest Ophthalmol Vis Sci. 2021 Oct 4;62(13):13. doi: 10.1167/iovs.62.13.13. Invest Ophthalmol Vis Sci. 2021. PMID: 34661608 Free PMC article.
-
Human Neural Progenitors Expressing GDNF Enhance Retinal Protection in a Rodent Model of Retinal Degeneration.Stem Cells Transl Med. 2023 Nov 3;12(11):727-744. doi: 10.1093/stcltm/szad054. Stem Cells Transl Med. 2023. PMID: 37786347 Free PMC article.
-
The Role of Retinal Pigment Epithelial Cells in Age-Related Macular Degeneration: Phagocytosis and Autophagy.Biomolecules. 2023 May 29;13(6):901. doi: 10.3390/biom13060901. Biomolecules. 2023. PMID: 37371481 Free PMC article. Review.
References
-
- Klein BE, Klein R, Linton KL. Intraocular pressure in an American community. The Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1992;33:2224–2228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

