Plumbagin induces RPE cell cycle arrest and apoptosis via p38 MARK and PI3K/AKT/mTOR signaling pathways in PVR
- PMID: 29534723
- PMCID: PMC5851073
- DOI: 10.1186/s12906-018-2155-3
Plumbagin induces RPE cell cycle arrest and apoptosis via p38 MARK and PI3K/AKT/mTOR signaling pathways in PVR
Abstract
Background: This study aimed to explore the effects of plumbagin (PLB) on ARPE-19 cells and underlying mechanism.
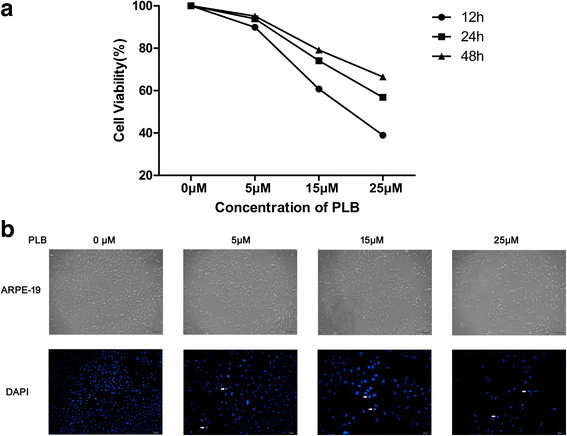
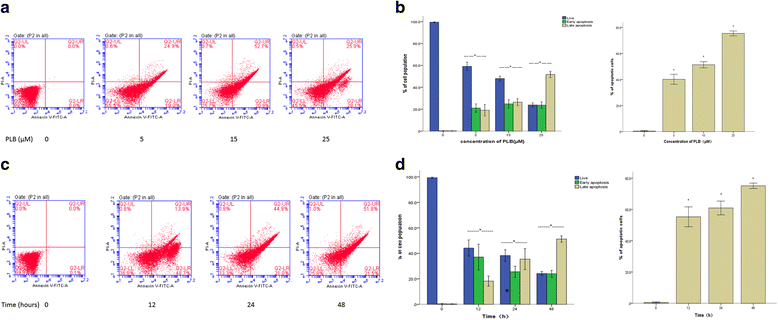
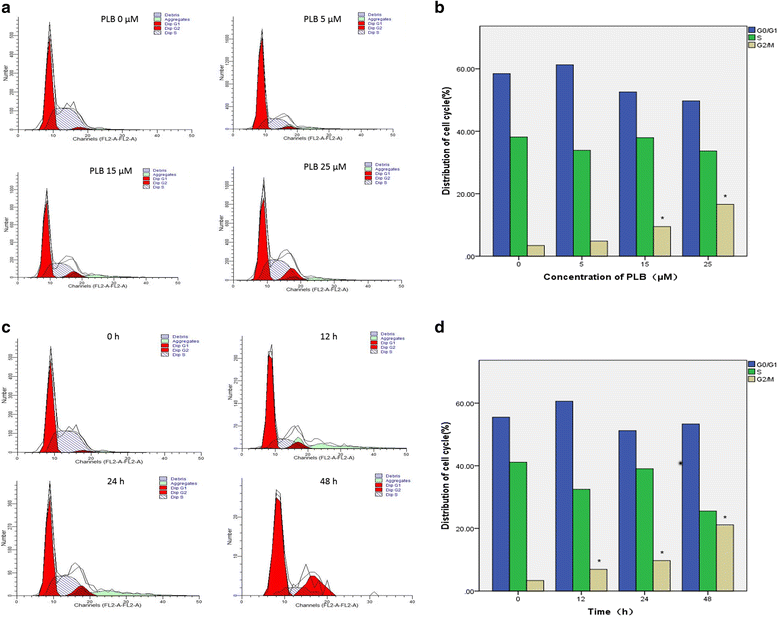
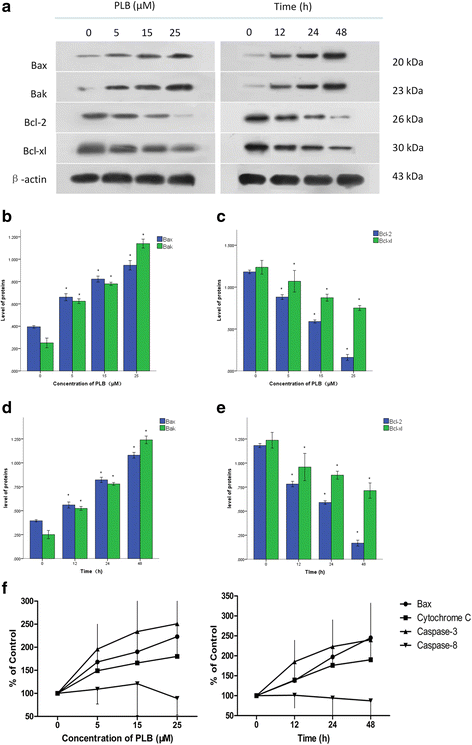
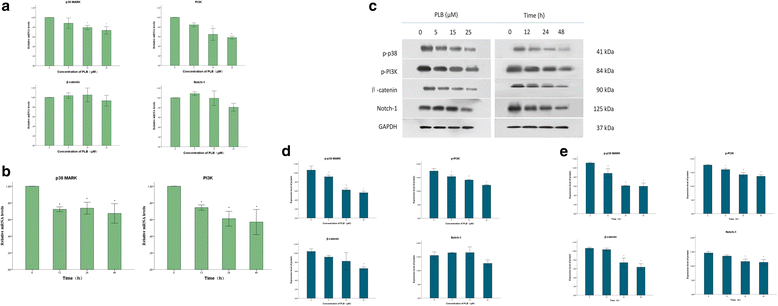
Methods: Cultured ARPE-19 cells were treated with various concentrations (0, 5, 15, and 25 μM) of PLB for 24 h or with 15 μM PLB for 12, 24 and 48 h. Then cell viability was evaluated by MTT assay and DAPI staining, while apoptosis and cell cycle progression of ARPE cells were assessed by flow cytometric analysis. Furthermore, the level of main regulatory proteins was examinated by Western boltting and the expression of relative mRNA was tested by Real-Time PCR.
Results: PLB exhibited potent inducing effects on cell cycle arrest at G2/M phase and apoptosis of ARPE cells via the modulation of Bcl-2 family regulators in a concentration- and time-dependent manner. PLB induced inhibition of phosphatidylinositol 3-kinase (PI3K) and p38 mitogen-activated protein kinase (p38 MAPK) signaling pathways contributing to the anti-proliferative activities in ARPE cells.
Conclusions: This is the first report to show that PLB could inhibit the proliferation of RPE cells through down-regulation of modulatory signaling pathways. The results open new avenues for the use of PLB in prevention and treatment of proliferative vitreoretinopathy.
Keywords: Plumbagin; Proliferation; RPE.
Conflict of interest statement
Authors’ information
Prof. JM is the deputy director of Ophthalmology Department of Second Hospital of Hebei Medical University, also a well known specialist majoring vitreoretinopathy in China. JA and QS are the experts of vitreoretinopathy who majors in therapy in PVR. HW and HC are fellows in this department. All members of this group have experience in experimental studies.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. All authors have agreed to authorship and order of authorship for this manuscript and that all authors have the appropriate permissions and rights to the reported data.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Asaria RH, Charteris DG. Proliferative vitreoretinopathy: developments in pathogenesis and treatment. Compr Ophthalmol Updat. 2006;7(4):179–185. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

