Vitamin A bio-modulates apoptosis via the mitochondrial pathway after hypoxic-ischemic brain damage
- PMID: 29534734
- PMCID: PMC5851324
- DOI: 10.1186/s13041-018-0360-0
Vitamin A bio-modulates apoptosis via the mitochondrial pathway after hypoxic-ischemic brain damage
Abstract
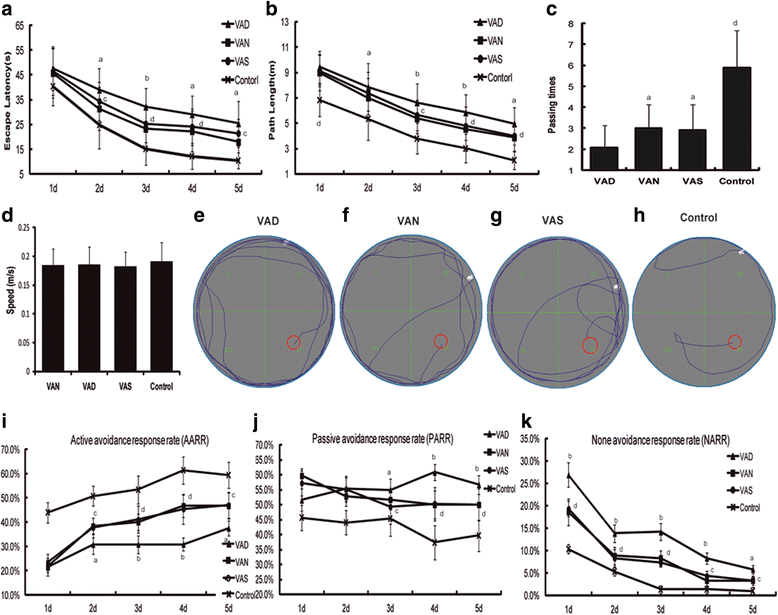
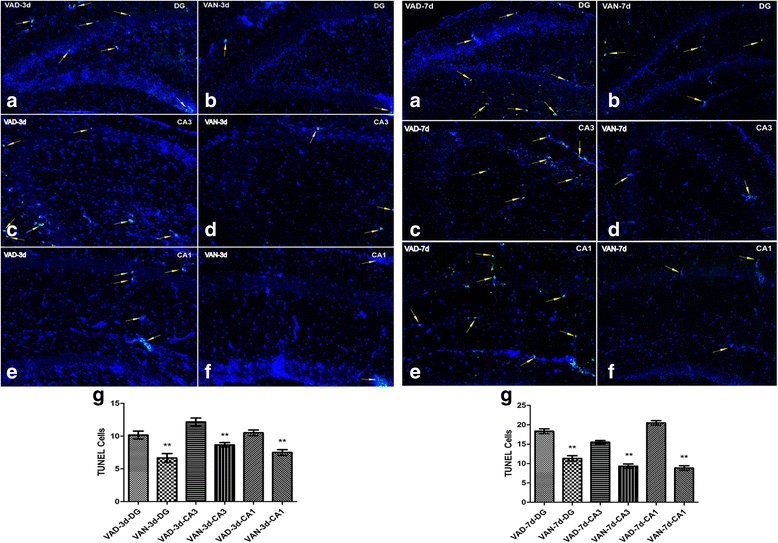
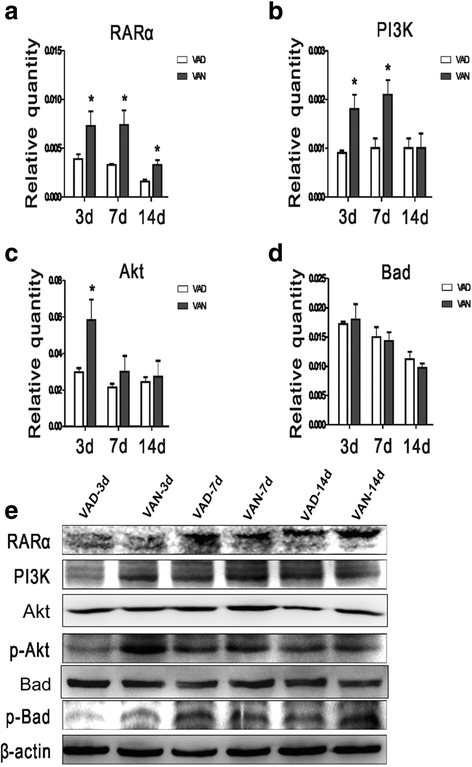
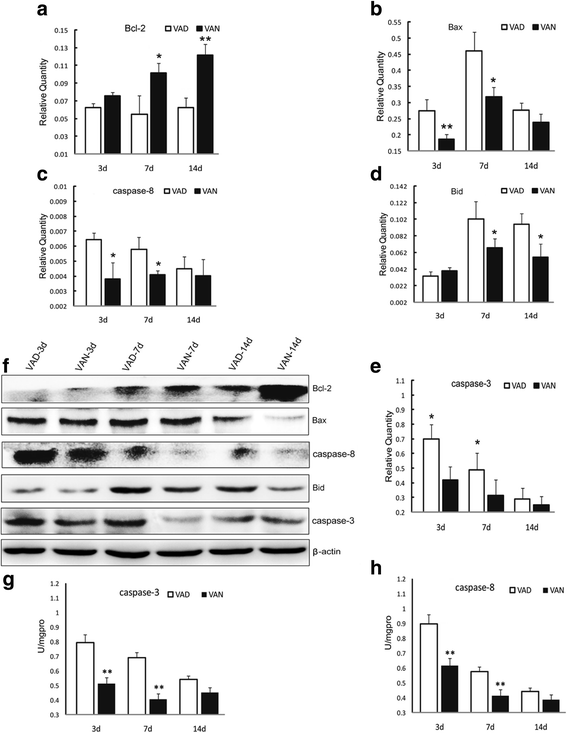
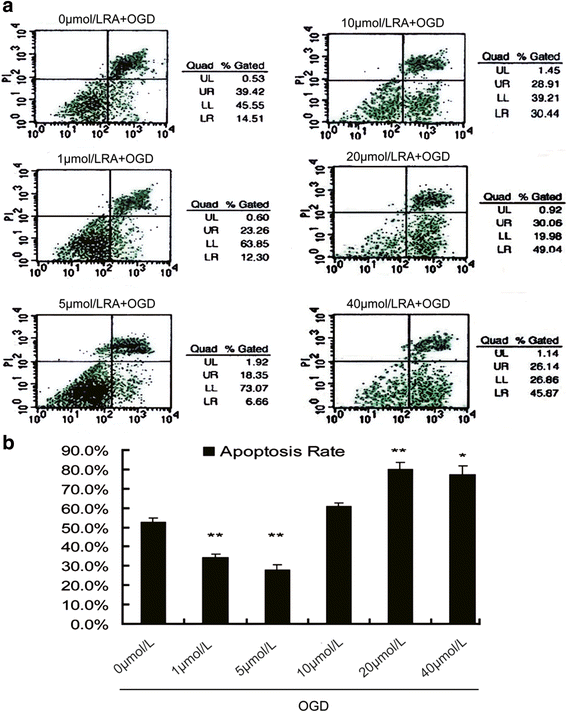
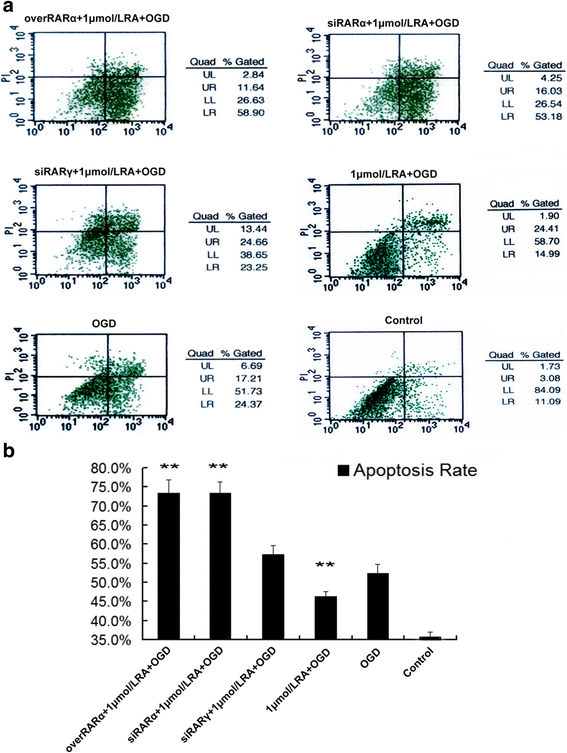
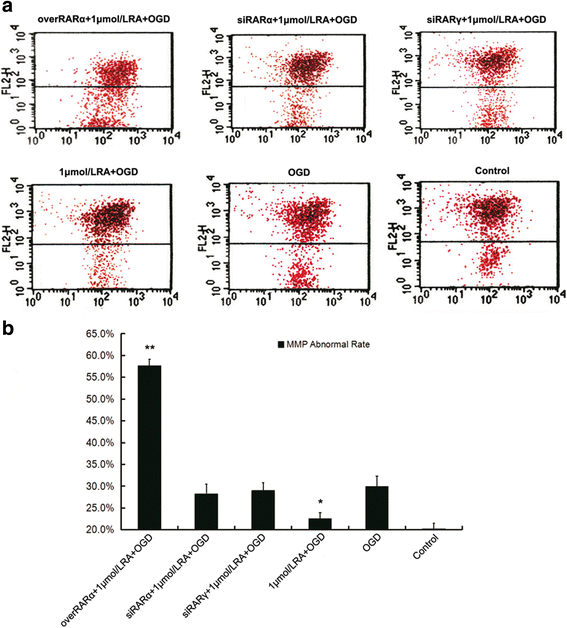
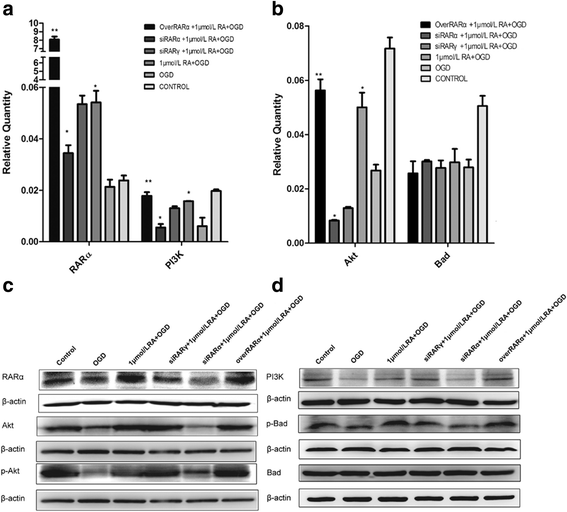
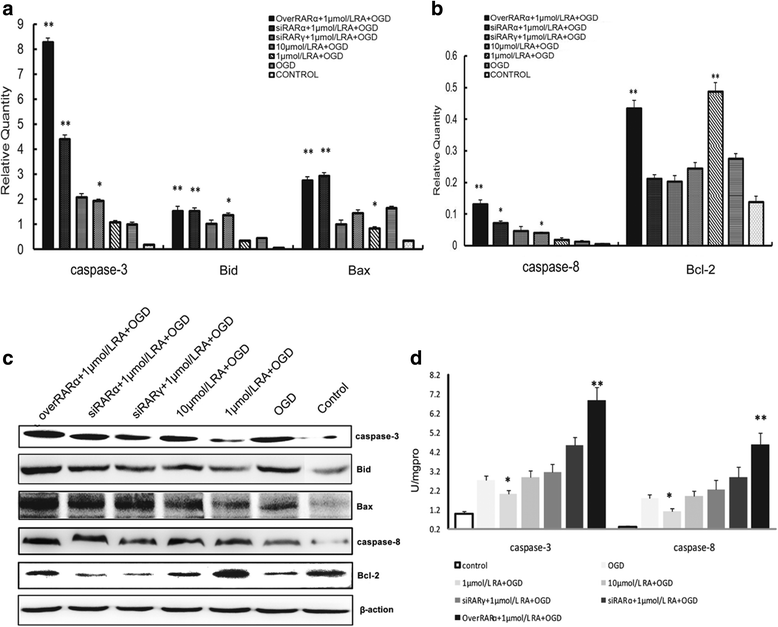
Our previous studies demonstrated that vitamin A deficiency (VAD) can impair the postnatal cognitive function of rats by damaging the hippocampus. The present study examined the effects of retinoic acid (RA) on apoptosis induced by hypoxic-ischemic damage in vivo and in vitro, and investigated the possible signaling pathway involved in the neuroprotective anti-apoptotic effects of RA. Flow cytometry, immunofluorescence staining and behavioral tests were used to evaluate the neuroprotective and anti-apoptotic effects of RA. The protein and mRNA levels of RARα, PI3K, Akt, Bad, caspase-3, caspase-8, Bcl-2, Bax, and Bid were measured with western blotting and real-time PCR, respectively. We found impairments in learning and spatial memory in VAD group compared with vitamin A normal (VAN) and vitamin A supplemented (VAS) group. Additionally, we showed that hippocampal apoptosis was weaker in the VAN group than that in VAD group. Relative to the VAD group, the VAN group also had increased mRNA and protein levels of RARα and PI3K, and upregulated phosphorylated Akt/Bad levels in vivo. In vitro, excessively low or high RA signaling promoted apoptosis. Furthermore, the effects on apoptosis involved the mitochondrial membrane potential (MMP). These data support the idea that sustained VAD following hypoxic-ischemic brain damage (HIBD) inhibits RARα, which downregulates the PI3K/Akt/Bad and Bcl-2/Bax pathways and upregulates the caspase-8/Bid pathway to influence the MMP, ultimately producing deficits in learning and spatial memory in adolescence. This suggests that clinical interventions for HIBD should include suitable doses of VA.
Keywords: Apoptosis; Hypoxic-ischemic brain damage (HIBD); Mitochondrial membrane potential (MMP); PI3K/Akt; Retinoic acid (RA); Vitamin A (VA).
Conflict of interest statement
Ethics approval
All animal experiments were approved by the Animal Experimentation Ethical Committee of the Zoology Center at Chongqing Medical University (Chongqing, China) [SCXK (Yu) 2012-0015].
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Vitamin A regulates neural stem cell proliferation in rats after hypoxic-ischemic brain damage via RARɑ-mediated modulation of the β-catenin pathway.Neurosci Lett. 2020 May 14;727:134922. doi: 10.1016/j.neulet.2020.134922. Epub 2020 Mar 20. Neurosci Lett. 2020. PMID: 32205185
-
Red photon treatment inhibits apoptosis via regulation of bcl-2 proteins and ROS levels, alleviating hypoxic-ischemic brain damage.Neuroscience. 2014 May 30;268:66-74. doi: 10.1016/j.neuroscience.2014.02.034. Epub 2014 Mar 5. Neuroscience. 2014. PMID: 24607343
-
Sevoflurane postconditioning improves long-term learning and memory of neonatal hypoxia-ischemia brain damage rats via the PI3K/Akt-mPTP pathway.Brain Res. 2016 Jan 1;1630:25-37. doi: 10.1016/j.brainres.2015.10.050. Epub 2015 Nov 2. Brain Res. 2016. PMID: 26541582
-
Vitamin A and Retinoic Acid in Cognition and Cognitive Disease.Annu Rev Nutr. 2020 Sep 23;40:247-272. doi: 10.1146/annurev-nutr-122319-034227. Annu Rev Nutr. 2020. PMID: 32966186 Review.
-
Role of mitochondria in apoptotic and necroptotic cell death in the developing brain.Clin Chim Acta. 2015 Dec 7;451(Pt A):35-8. doi: 10.1016/j.cca.2015.01.026. Epub 2015 Feb 4. Clin Chim Acta. 2015. PMID: 25661091 Free PMC article. Review.
Cited by
-
Gallic acid inhibits neuroinflammation and reduces neonatal hypoxic-ischemic brain damages.Front Pediatr. 2022 Dec 22;10:973256. doi: 10.3389/fped.2022.973256. eCollection 2022. Front Pediatr. 2022. PMID: 36619526 Free PMC article.
-
Administration of all-trans retinoic acid after experimental traumatic brain injury is brain protective.Br J Pharmacol. 2020 Nov;177(22):5208-5223. doi: 10.1111/bph.15259. Epub 2020 Oct 23. Br J Pharmacol. 2020. PMID: 32964418 Free PMC article.
-
Vitamin A Deficiency and the Lung.Nutrients. 2018 Aug 21;10(9):1132. doi: 10.3390/nu10091132. Nutrients. 2018. PMID: 30134568 Free PMC article. Review.
-
Proteome profiling of different rat brain regions reveals the modulatory effect of prolonged maternal separation on proteins involved in cell death-related processes.Biol Res. 2021 Feb 8;54(1):4. doi: 10.1186/s40659-021-00327-5. Biol Res. 2021. PMID: 33557947 Free PMC article.
-
Unraveling the causal pathway between phosphatidylinositol, metabolites, and metabolic syndrome: a Mendelian randomization study.Diabetol Metab Syndr. 2025 May 20;17(1):162. doi: 10.1186/s13098-025-01731-7. Diabetol Metab Syndr. 2025. PMID: 40394636 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

