Teriflunomide promotes oligodendroglial differentiation and myelination
- PMID: 29534752
- PMCID: PMC5851312
- DOI: 10.1186/s12974-018-1110-z
Teriflunomide promotes oligodendroglial differentiation and myelination
Abstract
Background: Multiple sclerosis (MS) is a neuroinflammatory autoimmune disease of the central nervous system (CNS) which in most cases initially presents with episodes of transient functional deficits (relapsing-remitting MS; RRMS) and eventually develops into a secondary progressive form (SPMS). Aside from neuroimmunological activities, MS is also characterized by neurodegenerative and regenerative processes. The latter involve the restoration of myelin sheaths-electrically insulating structures which are the primary targets of autoimmune attacks. Spontaneous endogenous remyelination takes place even in the adult CNS and is primarily mediated by activation, recruitment, and differentiation of resident oligodendroglial precursor cells (OPCs). However, the overall efficiency of remyelination is limited and further declines with disease duration and progression. From a therapeutic standpoint, it is therefore key to understand how oligodendroglial maturation can be modulated pharmacologically. Teriflunomide has been approved as a first-line treatment for RRMS in the USA and the European Union. As the active metabolite of leflunomide, an established disease-modifying anti-rheumatic drug, it mainly acts via an inhibition of de novo pyrimidine synthesis exerting a cytostatic effect on proliferating B and T cells.
Methods: We investigated teriflunomide-dependent effects on primary rat oligodendroglial homeostasis, proliferation, and differentiation related to cellular processes important for myelin repair hence CNS regeneration in vitro. To this end, several cellular parameters, including specific oligodendroglial maturation markers, in vitro myelination, and p53 family member signaling, were examined by means of gene/protein expression analyses. The rate of myelination was determined using neuron-oligodendrocyte co-cultures.
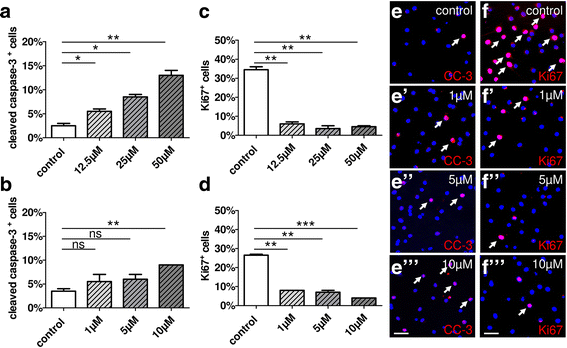
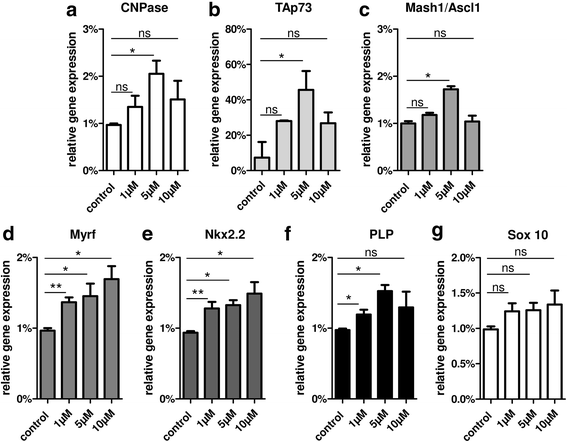
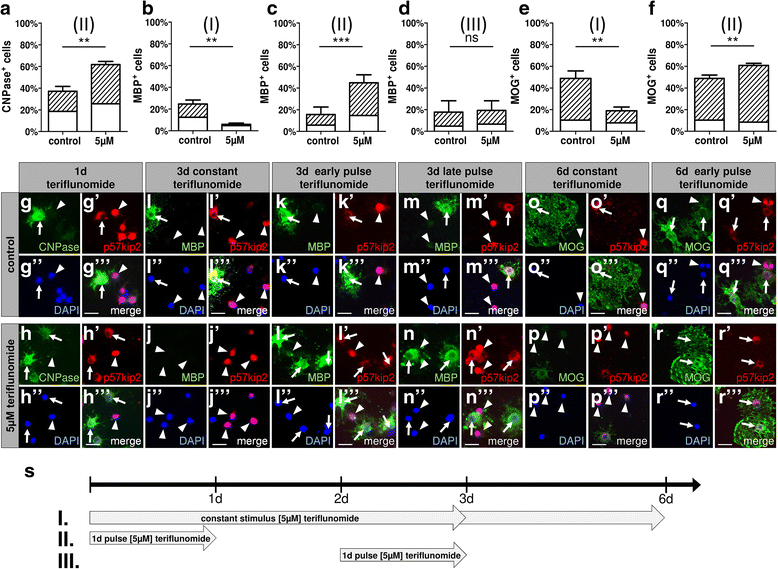
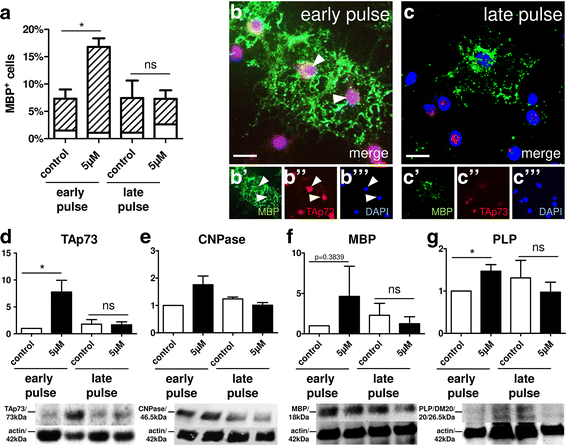
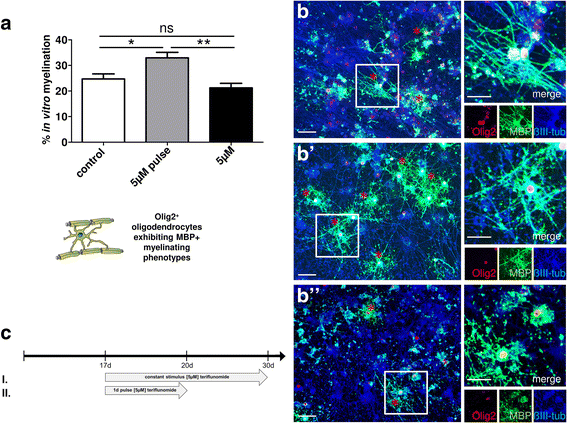
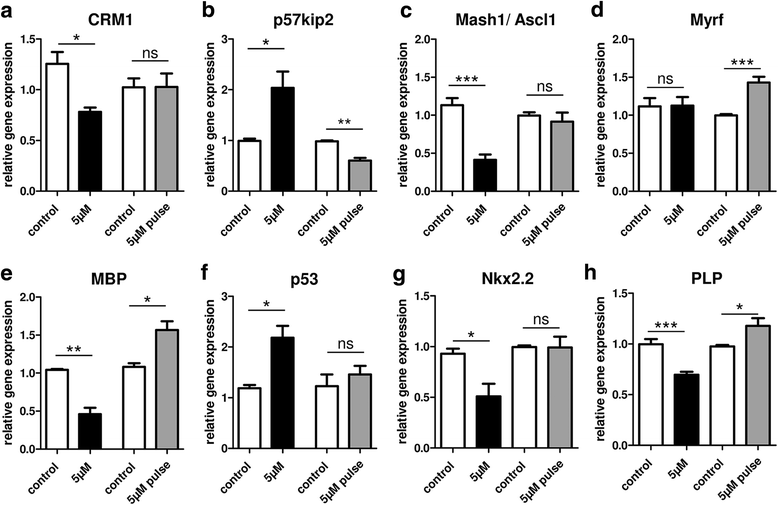
Results: Low teriflunomide concentrations resulted in cell cycle exit while higher doses led to decreased cell survival. Short-term teriflunomide pulses can efficiently promote oligodendroglial cell differentiation suggesting that young, immature cells could benefit from such stimulation. In vitro myelination can be boosted by means of an early stimulation window with teriflunomide. p73 signaling is functionally involved in promoting OPC differentiation and myelination.
Conclusion: Our findings indicate a critical window of opportunity during which regenerative oligodendroglial activities including myelination of CNS axons can be stimulated by teriflunomide.
Keywords: Inhibitor; Multiple sclerosis; Myelin repair; Neuroregeneration; Oligodendrocyte; Remyelination; Transcription factor; p57kip2; p73.
Conflict of interest statement
Ethics approval
Experimental procedures were approved by the Institutional Animal Care and Use Committee of the Heinrich Heine University in accordance with the criteria outlined in the Institutional Guidelines for Animal Research.
Consent for publication
Not applicable.
Competing interests
AM and LR have no competing interests. PG and PK performed consultancy work for Geneuro. DK received compensation for speaking from Grifols SA. HPH received compensation for consulting, speaking, and serving on steering committees from Bayer Healthcare, Biogen, Geneuro, MedImmune, Merck, Novartis, Opexa, Receptos Celgene, Roche, Sanofi Genzyme, and Teva with the approval by the Rector of Heinrich Heine University.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Bruneau JM, Yea CM, Spinella-Jaegle S, Fudali C, Woodward K, Robson PA, Sautes C, Westwood R, Kuo EA, Williamson RA, Ruuth E. Purification of human dihydro-orotate dehydrogenase and its inhibition by A77 1726, the active metabolite of leflunomide. Biochem J. 1998;336(Pt 2):299–303. doi: 10.1042/bj3360299. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

