Single- and Multi-Fraction Stereotactic Radiosurgery Dose Tolerances of the Optic Pathways
- PMID: 29534899
- PMCID: PMC9479557
- DOI: 10.1016/j.ijrobp.2018.01.053
Single- and Multi-Fraction Stereotactic Radiosurgery Dose Tolerances of the Optic Pathways
Abstract
Purpose: Dosimetric and clinical predictors of radiation-induced optic nerve/chiasm neuropathy (RION) after single-fraction stereotactic radiosurgery (SRS) or hypofractionated (2-5 fractions) radiosurgery (fSRS) were analyzed from pooled data that were extracted from published reports (PubMed indexed from 1990 to June 2015). This study was undertaken as part of the American Association of Physicists in Medicine Working Group on Stereotactic Body Radiotherapy, investigating normal tissue complication probability (NTCP) after hypofractionated radiation.
Methods and materials: Eligible studies described dose delivered to optic nerve/chiasm and provided crude or actuarial toxicity risks, with visual endpoints (ie, loss of visual acuity, alterations in visual fields, and/or blindness/complete vision loss). Studies of patients with optic nerve sheath tumors, optic nerve gliomas, or ocular/uveal melanoma were excluded to obviate direct tumor effects on visual outcomes, as were studies not specifying causes of vision loss (ie, tumor progression vs RION).
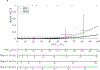
Results: Thirty-four studies (1578 patients) were analyzed. Histologies included pituitary adenoma, cavernous sinus meningioma, craniopharyngioma, and malignant skull base tumors. Prior resection (76% of patients) did not correlate with RION risk (P = .66). Prior irradiation (6% of patients) was associated with a crude 10-fold increased RION risk versus no prior radiation therapy. In patients with no prior radiation therapy receiving SRS/fSRS in 1-5 fractions, optic apparatus maximum point doses resulting in <1% RION risks include 12 Gy in 1 fraction (which is greater than our recommendation of 10 Gy in 1 fraction), 20 Gy in 3 fractions, and 25 Gy in 5 fractions. Omitting multi-fraction data (and thereby eliminating uncertainties associated with dose conversions), a single-fraction dose of 10 Gy was associated with a 1% RION risk. Insufficient details precluded modeling of NTCP risks after prior radiation therapy.
Conclusions: Optic apparatus NTCP and tolerance doses after single- and multi-fraction stereotactic radiosurgery are presented. Additional standardized dosimetric and toxicity reporting is needed to facilitate future pooled analyses and better define RION NTCP after SRS/fSRS.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest:
Figures




References
-
- Aans/cns/astro definition of stereotactic radiosurgery http://www.aans.org/-/media/Files/AANS/About-Us/Position-Statements/fiel..., 2006.
-
- Lent soma scales for all anatomic sites. Int J Radiat Oncol Biol Phys 1995;31:1049–1091. - PubMed
-
- Nih publication no. 09–5410: Http://evs.Nci.Nih.Gov/ftp1/ctcae/ctcae_4.03_2010-06-14_quickreference_8..., 2009.
-
- Adler JR Jr., et al. Visual field preservation after multisession cyberknife radiosurgery for perioptic lesions. Neurosurgery 2006;59:244–254; discussion 244–254. - PubMed
-
- Agresti A, Coull BA. Approximate is better than “exact” for interval estimation of binomial proportiions. The Am. Statistician 1998;52:119–126.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

