Filgrastim enhances T-cell clearance by antithymocyte globulin exposure after unrelated cord blood transplantation
- PMID: 29535105
- PMCID: PMC5851423
- DOI: 10.1182/bloodadvances.2017015487
Filgrastim enhances T-cell clearance by antithymocyte globulin exposure after unrelated cord blood transplantation
Abstract
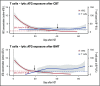
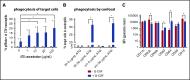
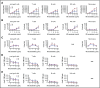
Residual antithymocyte globulin (ATG; Thymoglobulin) exposure after allogeneic hematopoietic (stem) cell transplantation (HCT) delays CD4+ T-cell immune reconstitution (CD4+ IR), subsequently increasing morbidity and mortality. This effect seems particularly present after cord blood transplantation (CBT) compared to bone marrow transplantation (BMT). The reason for this is currently unknown. We investigated the effect of active-ATG exposure on CD4+ IR after BMT and CBT in 275 patients (CBT n = 155, BMT n = 120; median age, 7.8 years; range, 0.16-19.2 years) receiving their first allogeneic HCT between January 2008 and September 2016. Multivariate log-rank tests (with correction for covariates) revealed that CD4+ IR was faster after CBT than after BMT with <10 active-ATG × day/mL (P = .018) residual exposure. In contrast, >10 active-ATG × day/mL exposure severely impaired CD4+ IR after CBT (P < .001), but not after BMT (P = .74). To decipher these differences, we performed ATG-binding and ATG-cytotoxicity experiments using cord blood- and bone marrow graft-derived T-cell subsets, B cells, natural killer cells, and monocytes. No differences were observed. Nevertheless, a major covariate in our cohort was Filgrastim treatment (only given after CBT). We found that Filgrastim (granulocyte colony-stimulating factor [G-CSF]) exposure highly increased neutrophil-mediated ATG cytotoxicity (by 40-fold [0.5 vs 20%; P = .002]), which explained the enhanced T-cell clearance after CBT. These findings imply revision of the use (and/or timing) of G-CSF in patients with residual ATG exposure.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures



Similar articles
-
Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis.Lancet Haematol. 2015 May;2(5):e194-203. doi: 10.1016/S2352-3026(15)00045-9. Epub 2015 Apr 21. Lancet Haematol. 2015. PMID: 26688094
-
Excellent T-cell reconstitution and survival depend on low ATG exposure after pediatric cord blood transplantation.Blood. 2016 Dec 8;128(23):2734-2741. doi: 10.1182/blood-2016-06-721936. Epub 2016 Oct 4. Blood. 2016. PMID: 27702800 Clinical Trial.
-
Early and Long-Term Impaired T Lymphocyte Immune Reconstitution after Cord Blood Transplantation with Antithymocyte Globulin.Biol Blood Marrow Transplant. 2017 Mar;23(3):491-497. doi: 10.1016/j.bbmt.2016.11.014. Epub 2016 Nov 22. Biol Blood Marrow Transplant. 2017. PMID: 27888015
-
Sufficient Immunosuppression with Thymoglobulin Is Essential for a Successful Haplo-Myeloid Bridge in Haploidentical-Cord Blood Transplantation.Biol Blood Marrow Transplant. 2015 Oct;21(10):1839-45. doi: 10.1016/j.bbmt.2015.06.001. Epub 2015 Jun 26. Biol Blood Marrow Transplant. 2015. PMID: 26119367
-
Antithymocyte globulin in allogeneic hematopoietic cell transplantation: benefits and limitations.Immunotherapy. 2016;8(4):435-47. doi: 10.2217/imt.15.128. Immunotherapy. 2016. PMID: 26973125 Review.
Cited by
-
Targeting the high affinity receptor, FcγRI, in autoimmune disease, neuropathy, and cancer.Immunother Adv. 2022 May 28;2(1):ltac011. doi: 10.1093/immadv/ltac011. eCollection 2022. Immunother Adv. 2022. PMID: 36284837 Free PMC article. Review.
-
Robust CD4+ T-cell recovery in adults transplanted with cord blood and no antithymocyte globulin.Blood Adv. 2020 Jan 14;4(1):191-202. doi: 10.1182/bloodadvances.2019000836. Blood Adv. 2020. PMID: 31935291 Free PMC article.
-
Cord blood power and the definition of success after BMT.Blood Adv. 2023 May 9;7(9):1811-1812. doi: 10.1182/bloodadvances.2022009178. Blood Adv. 2023. PMID: 36350719 Free PMC article. No abstract available.
-
Post-transplant G-CSF impedes engraftment of gene-edited human hematopoietic stem cells by exacerbating p53-mediated DNA damage response.Cell Stem Cell. 2025 Jan 2;32(1):53-70.e8. doi: 10.1016/j.stem.2024.10.013. Epub 2024 Nov 12. Cell Stem Cell. 2025. PMID: 39536761
-
Planned Granulocyte Colony-Stimulating Factor Adversely Impacts Survival after Allogeneic Hematopoietic Cell Transplantation Performed with Thymoglobulin for Myeloid Malignancy.Transplant Cell Ther. 2021 Dec;27(12):993.e1-993.e8. doi: 10.1016/j.jtct.2021.08.031. Epub 2021 Oct 2. Transplant Cell Ther. 2021. PMID: 34507002 Free PMC article.
References
-
- Bartelink IH, Belitser SV, Knibbe CA, et al. . Immune reconstitution kinetics as an early predictor for mortality using various hematopoietic stem cell sources in children. Biol Blood Marrow Transplant. 2013;19(2):305-313. - PubMed
-
- Berger M, Figari O, Bruno B, et al. . Lymphocyte subsets recovery following allogeneic bone marrow transplantation (BMT): CD4+ cell count and transplant-related mortality. Bone Marrow Transplant. 2008;41(1):55-62. - PubMed
-
- Kim DH, Sohn SK, Won DI, Lee NY, Suh JS, Lee KB. Rapid helper T-cell recovery above 200 x 10 6/l at 3 months correlates to successful transplant outcomes after allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;37(12):1119-1128. - PubMed
-
- Pourgheysari B, Piper KP, McLarnon A, et al. . Early reconstitution of effector memory CD4+ CMV-specific T cells protects against CMV reactivation following allogeneic SCT. Bone Marrow Transplant. 2009;43(11):853-861. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

