Serum High-Mobility-Group Box 1 as a Biomarker and a Therapeutic Target during Respiratory Virus Infections
- PMID: 29535197
- PMCID: PMC5850323
- DOI: 10.1128/mBio.00246-18
Serum High-Mobility-Group Box 1 as a Biomarker and a Therapeutic Target during Respiratory Virus Infections
Abstract
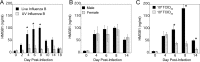
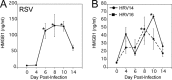
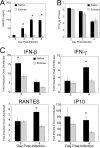
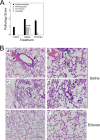
Host-derived "danger-associated molecular patterns" (DAMPs) contribute to innate immune responses and serve as markers of disease progression and severity for inflammatory and infectious diseases. There is accumulating evidence that generation of DAMPs such as oxidized phospholipids and high-mobility-group box 1 (HMGB1) during influenza virus infection leads to acute lung injury (ALI). Treatment of influenza virus-infected mice and cotton rats with the Toll-like receptor 4 (TLR4) antagonist Eritoran blocked DAMP accumulation and ameliorated influenza virus-induced ALI. However, changes in systemic HMGB1 kinetics during the course of influenza virus infection in animal models and humans have yet to establish an association of HMGB1 release with influenza virus infection. To this end, we used the cotton rat model that is permissive to nonadapted strains of influenza A and B viruses, respiratory syncytial virus (RSV), and human rhinoviruses (HRVs). Serum HMGB1 levels were measured by an enzyme-linked immunosorbent assay (ELISA) prior to infection until day 14 or 18 post-infection. Infection with either influenza A or B virus resulted in a robust increase in serum HMGB1 levels that decreased by days 14 to 18. Inoculation with the live attenuated vaccine FluMist resulted in HMGB1 levels that were significantly lower than those with infection with live influenza viruses. RSV and HRVs showed profiles of serum HMGB1 induction that were consistent with their replication and degree of lung pathology in cotton rats. We further showed that therapeutic treatment with Eritoran of cotton rats infected with influenza B virus significantly blunted serum HMGB1 levels and improved lung pathology, without inhibiting virus replication. These findings support the use of drugs that block HMGB1 to combat influenza virus-induced ALI.IMPORTANCE Influenza virus is a common infectious agent causing serious seasonal epidemics, and there is urgent need to develop an alternative treatment modality for influenza virus infection. Recently, host-derived DAMPs, such as oxidized phospholipids and HMGB1, were shown to be generated during influenza virus infection and cause ALI. To establish a clear link between influenza virus infection and HMGB1 as a biomarker, we have systematically analyzed temporal patterns of serum HMGB1 release in cotton rats infected with nonadapted strains of influenza A and B viruses and compared these patterns with a live attenuated influenza vaccine and infection by other respiratory viruses. Towards development of a new therapeutic modality, we show herein that blocking serum HMGB1 levels by Eritoran improves lung pathology in influenza B virus-infected cotton rats. Our study is the first report of systemic HMGB1 as a potential biomarker of severity in respiratory virus infections and confirms that drugs that block virus-induced HMGB1 ameliorate ALI.
Keywords: HMGB1; biomarker; influenza; respiratory syncytial virus; respiratory viruses.
Copyright © 2018 Patel et al.
Figures

References
-
- Su S, Chaves SS, Perez A, D’Mello T, Kirley PD, Yousey-Hindes K, Farley MM, Harris M, Sharangpani R, Lynfield R, Morin C, Hancock EB, Zansky S, Hollick GE, Fowler B, McDonald-Hamm C, Thomas A, Horan V, Lindegren ML, Schaffner W, Price A, Bandyopadhyay A, Fry AM. 2014. Comparing clinical characteristics between hospitalized adults with laboratory-confirmed influenza A and B virus infection. Clin Infect Dis 59:252–255. doi:10.1093/cid/ciu269. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

