Human Memory B Cells Targeting Staphylococcus aureus Exotoxins Are Prevalent with Skin and Soft Tissue Infection
- PMID: 29535203
- PMCID: PMC5850327
- DOI: 10.1128/mBio.02125-17
Human Memory B Cells Targeting Staphylococcus aureus Exotoxins Are Prevalent with Skin and Soft Tissue Infection
Abstract
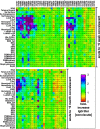
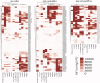
Staphylococcus aureus is a Gram-positive opportunistic pathogen that causes superficial and invasive infections in the hospital and community. High mortality from infection emphasizes the need for improved methods for prevention and treatment. Although S. aureus possesses an arsenal of virulence factors that contribute to evasion of host defenses, few studies have examined long-term humoral and B-cell responses. Adults with acute-phase skin and soft tissue infections were recruited; blood samples were obtained; and S. aureus isolates, including methicillin-resistant strains, were subjected to genomic sequence analysis. In comparisons of acute-phase sera with convalescent-phase sera, a minority (37.5%) of patients displayed 2-fold or greater increases in antibody titers against three or more S. aureus antigens, whereas nearly half exhibited no changes, despite the presence of toxin genes in most infecting strains. Moreover, enhanced antibody responses waned over time, which could reflect a defect in B-cell memory or long-lived plasma cells. However, memory B cells reactive with a range of S. aureus antigens were prevalent at both acute-phase and convalescent-phase time points. While some memory B cells exhibited toxin-specific binding, those cross-reactive with structurally related leucocidin subunits were dominant across patients, suggesting the targeting of conserved epitopes. Memory B-cell reactivity correlated with serum antibody levels for selected S. aureus exotoxins, suggesting a relationship between the cellular and humoral compartments. Overall, although there was no global defect in the representation of anti-S. aureus memory B cells, there was evidence of restrictions in the range of epitopes recognized, which may suggest potential therapeutic approaches for augmenting host defenses.IMPORTANCE The contribution of B-cell memory and long-term antibody responses to host defenses against S. aureus exotoxins remains poorly understood. Our studies confirmed that infection did not commonly lead to enhanced long-term humoral responses. Whereas circulating memory B cells against S. aureus secreted exotoxins were prevalent, they were dominated by cross-reactivity with structurally related leucocidin subunits, consistent with recognition of conserved epitopes. These findings also provide the first evidence of a relationship between the reactivity of antistaphylococcal circulating memory B cells and serum antibody levels. In general, infection was not associated with a global defect in B-cell memory for S. aureus secreted factors, and responses were highly dominated by cross-reactivity to structurally related exotoxins, which arguably may alone be suboptimal in providing host defenses. Our studies illuminate aspects of the S. aureus-host relationship that may better inform strategies for the development of an effective protective vaccine.
Keywords: MRSA; Staphylococcus aureus; antibodies; host response; host-pathogen interactions; leukocidins; memory B cells; pore-forming toxins; skin and soft tissue infection (SSTI); superantigens.
Copyright © 2018 Pelzek et al.
Figures



References
-
- Lee BY, Singh A, David MZ, Bartsch SM, Slayton RB, Huang SS, Zimmer SM, Potter MA, Macal CM, Lauderdale DS, Miller LG, Daum RS. 2013. The economic burden of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). Clin Microbiol Infect 19:528–536. doi:10.1111/j.1469-0691.2012.03914.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

