Discovery and characterization of a sulfoquinovose mutarotase using kinetic analysis at equilibrium by exchange spectroscopy
- PMID: 29535276
- PMCID: PMC5902678
- DOI: 10.1042/BCJ20170947
Discovery and characterization of a sulfoquinovose mutarotase using kinetic analysis at equilibrium by exchange spectroscopy
Abstract
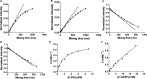
Bacterial sulfoglycolytic pathways catabolize sulfoquinovose (SQ), or glycosides thereof, to generate a three-carbon metabolite for primary cellular metabolism and a three-carbon sulfonate that is expelled from the cell. Sulfoglycolytic operons encoding an Embden-Meyerhof-Parnas-like or Entner-Doudoroff (ED)-like pathway harbor an uncharacterized gene (yihR in Escherichia coli; PpSQ1_00415 in Pseudomonas putida) that is up-regulated in the presence of SQ, has been annotated as an aldose-1-epimerase and which may encode an SQ mutarotase. Our sequence analyses and structural modeling confirmed that these proteins possess mutarotase-like active sites with conserved catalytic residues. We overexpressed the homolog from the sulfo-ED operon of Herbaspirillum seropedicaea (HsSQM) and used it to demonstrate SQ mutarotase activity for the first time. This was accomplished using nuclear magnetic resonance exchange spectroscopy, a method that allows the chemical exchange of magnetization between the two SQ anomers at equilibrium. HsSQM also catalyzed the mutarotation of various aldohexoses with an equatorial 2-hydroxy group, including d-galactose, d-glucose, d-glucose-6-phosphate (Glc-6-P), and d-glucuronic acid, but not d-mannose. HsSQM displayed only 5-fold selectivity in terms of efficiency (kcat/KM) for SQ versus the glycolysis intermediate Glc-6-P; however, its proficiency [kuncat/(kcat/KM)] for SQ was 17 000-fold better than for Glc-6-P, revealing that HsSQM preferentially stabilizes the SQ transition state.
Keywords: NMR spectroscopy; enzymology; metabolism; mutarotase; sulfoglycolysis.
© 2018 The Author(s).
Conflict of interest statement
The Authors declare that there are no competing interests associated with the manuscript.
Figures





References
-
- Tanner M. (1997) Mutarotases In Comprehensive Biological Catalysis (Sinnott M. L., ed.), pp. 208–209, Academic Press, London
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

