Organ-specific responses during brain death: increased aerobic metabolism in the liver and anaerobic metabolism with decreased perfusion in the kidneys
- PMID: 29535334
- PMCID: PMC5849719
- DOI: 10.1038/s41598-018-22689-9
Organ-specific responses during brain death: increased aerobic metabolism in the liver and anaerobic metabolism with decreased perfusion in the kidneys
Abstract
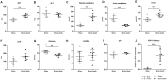
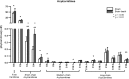
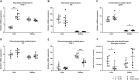
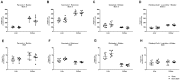
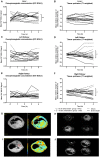
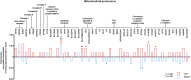
Hepatic and renal energy status prior to transplantation correlates with graft survival. However, effects of brain death (BD) on organ-specific energy status are largely unknown. We studied metabolism, perfusion, oxygen consumption, and mitochondrial function in the liver and kidneys following BD. BD was induced in mechanically-ventilated rats, inflating an epidurally-placed Fogarty-catheter, with sham-operated rats as controls. A 9.4T-preclinical MRI system measured hourly oxygen availability (BOLD-related R2*) and perfusion (T1-weighted). After 4 hrs, tissue was collected, mitochondria isolated and assessed with high-resolution respirometry. Quantitative proteomics, qPCR, and biochemistry was performed on stored tissue/plasma. Following BD, the liver increased glycolytic gene expression (Pfk-1) with decreased glycogen stores, while the kidneys increased anaerobic- (Ldha) and decreased gluconeogenic-related gene expression (Pck-1). Hepatic oxygen consumption increased, while renal perfusion decreased. ATP levels dropped in both organs while mitochondrial respiration and complex I/ATP synthase activity were unaffected. In conclusion, the liver responds to increased metabolic demands during BD, enhancing aerobic metabolism with functional mitochondria. The kidneys shift towards anaerobic energy production while renal perfusion decreases. Our findings highlight the need for an organ-specific approach to assess and optimise graft quality prior to transplantation, to optimise hepatic metabolic conditions and improve renal perfusion while supporting cellular detoxification.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- National Data: Donors Recovered in the U.S. by Donor Type. Available at: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/# (Accessed: 1st March 2017).
-
- Annual Report 2015. Available at: https://eurotransplant.org/cms/mediaobject.php?file=AR_ET_20153.pdf (Accessed: 1st March 2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

