Direct coupling of detergent purified human mGlu5 receptor to the heterotrimeric G proteins Gq and Gs
- PMID: 29535347
- PMCID: PMC5849714
- DOI: 10.1038/s41598-018-22729-4
Direct coupling of detergent purified human mGlu5 receptor to the heterotrimeric G proteins Gq and Gs
Abstract
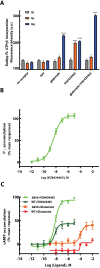
The metabotropic glutamate (mGlu) receptors are class C G protein-coupled receptors (GPCRs) that modulate synaptic activity and plasticity throughout the mammalian brain. Signal transduction is initiated by glutamate binding to the venus flytrap domains (VFT), which initiates a conformational change that is transmitted to the conserved heptahelical domains (7TM) and results ultimately in the activation of intracellular G proteins. While both mGlu1 and mGlu5 activate Gαq G-proteins, they also increase intracellular cAMP concentration through an unknown mechanism. To study directly the G protein coupling properties of the human mGlu5 receptor homodimer, we purified the full-length receptor, which required careful optimisation of the expression, N-glycosylation and purification. We successfully purified functional mGlu5 that activated the heterotrimeric G protein Gq. The high-affinity agonist-PAM VU0424465 also activated the purified receptor in the absence of an orthosteric agonist. In addition, it was found that purified mGlu5 was capable of activating the G protein Gs either upon stimulation with VU0424465 or glutamate, although the later induced a much weaker response. Our findings provide important mechanistic insights into mGlu5 G protein-dependent activity and selectivity.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

