Mesenchymal stem cells in multiple myeloma: a therapeutical tool or target?
- PMID: 29535427
- PMCID: PMC6035148
- DOI: 10.1038/s41375-018-0061-9
Mesenchymal stem cells in multiple myeloma: a therapeutical tool or target?
Abstract
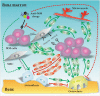
Multiple myeloma (MM) is a malignant plasma cell (PC) disorder, characterized by a complex interactive network of tumour cells and the bone marrow (BM) stromal microenvironment, contributing to MM cell survival, proliferation and chemoresistance. Mesenchymal stem cells (MSCs) represent the predominant stem cell population of the bone marrow stroma, capable of differentiating into multiple cell lineages, including fibroblasts, adipocytes, chondrocytes and osteoblasts. MSCs can migrate towards primary tumours and metastatic sites, implying that these cells might modulate tumour growth and metastasis. However, this issue remains controversial and is not well understood. Interestingly, several recent studies have shown functional abnormalities of MM patient-derived MSCs indicating that MSCs are not just by-standers in the BM microenvironment but rather active players in the pathophysiology of this disease. It appears that the complex interaction of MSCs and MM cells is critical for MM development and disease outcome. This review will focus on the current understanding of the biological role of MSCs in MM as well as the potential utility of MSC-based therapies in this malignancy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Arnulf B, Lecourt S, Soulier J, Ternaux B, Lacassagne MN, Crinquette A, et al. Phenotypic and functional characterization of bone marrow mesenchymal stem cells derived from patients with multiple myeloma. Leukemia. 2007;21:158–63. - PubMed
-
- Garderet L, Mazurier C, Chapel A, Ernou I, Boutin L, Holy X, et al. Mesenchymal stem cell abnormalities in patients with multiple myeloma. Leuk Lymphoma. 2007;48:2032–41. - PubMed
-
- Wallace SR, Oken MM, Lunetta KL, Panoskaltsis-Mortari A, Masellis AM. Abnormalities of bone marrow mesenchymal cells in multiple myeloma patients. Cancer. 2001;91:1219–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

