Bioequivalence of HTX-019 (aprepitant IV) and fosaprepitant in healthy subjects: a Phase I, open-label, randomized, two-way crossover evaluation
- PMID: 29535504
- PMCID: PMC5837372
- DOI: 10.2147/DDDT.S155875
Bioequivalence of HTX-019 (aprepitant IV) and fosaprepitant in healthy subjects: a Phase I, open-label, randomized, two-way crossover evaluation
Abstract
Introduction: Fosaprepitant, an intravenous (IV) aprepitant prodrug for chemotherapy-induced nausea and vomiting prophylaxis, is associated with systemic and infusion-site reactions attributed in part to its surfactant, polysorbate 80. HTX-019 is an IV aprepitant formulation free of polysorbate 80 and other synthetic surfactants.
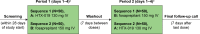
Materials and methods: This open-label, single-dose, randomized, two-way crossover bioequivalence study compared pharmacokinetics and safety of HTX-019 and fosaprepitant. Healthy subjects received single-dose HTX-019 (130 mg) or fosaprepitant (150 mg) IV over 30 min, with ≥7-day washout between doses. Blood samples were evaluated for pharmacokinetics and bioequivalence; safety evaluation included treatment-emergent adverse events (TEAEs) and serious adverse events. Ninety-seven of one hundred enrolled subjects completed the study.
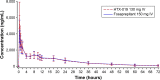
Results: Baseline characteristics were comparable between treatment sequences. For HTX-019, mean (percent coefficient of variation) area under the curve (AUC) from time 0 to time of last measurable plasma concentration (AUC0-t), AUC from time 0 to infinity (AUC0-inf), and plasma concentration at 12 h (C12 h) for HTX-019 were 43,729 h*ng/mL (32.7), 45,460 h*ng/mL (36.8), and 988.4 ng/mL (27.5), respectively; corresponding fosaprepitant values were 44,130 h*ng/mL (32.0), 46,163 h*ng/mL (36.6), and 1,022 ng/mL (28.5). Also, 90% CIs (94.186-101.354) were within bioequivalence bounds (80%-125%). Within 1 h following infusion start, one (1%) HTX-019 recipient reported one TEAE, while 20 (20%) fosaprepitant recipients reported 32 TEAEs. Dyspnea occurred in three fosaprepitant recipients (at <1 min in two subjects and at 18 min in one subject, considered study drug related) and one HTX-019 recipient (at 120 h, associated with a respiratory tract infection and considered not related to the study drug). No severe TEAEs, serious adverse events, or deaths occurred; all TEAEs resolved.
Conclusion: HTX-019 was bioequivalent to fosaprepitant and may provide a safer alternative to fosaprepitant for chemotherapy-induced nausea and vomiting prophylaxis.
Keywords: antiemetics; polysorbate 80; safety; surfactant.
Conflict of interest statement
Disclosure TO, MRK, MC, NC, and BQ are Heron Therapeutics, Inc., employees and report stock ownership. TO, NC, and BQ report leadership roles with Heron Therapeutics, Inc. TO reports travel, accommodation, and expenses reimbursed by Heron Therapeutics, Inc. The authors report no other conflicts of interest in this work.
Figures

Similar articles
-
Safety of HTX-019 (intravenous aprepitant) and fosaprepitant in healthy subjects.Future Oncol. 2018 Nov;14(27):2849-2859. doi: 10.2217/fon-2018-0311. Epub 2018 Jun 6. Future Oncol. 2018. PMID: 29873529 Clinical Trial.
-
HTX-019 via 2-min injection or 30-min infusion in healthy subjects.Future Oncol. 2019 Mar;15(8):865-874. doi: 10.2217/fon-2018-0809. Epub 2018 Dec 21. Future Oncol. 2019. PMID: 30574797 Clinical Trial.
-
Tolerability of fosaprepitant and bioequivalency to aprepitant in healthy subjects.J Clin Pharmacol. 2007 Jul;47(7):834-40. doi: 10.1177/0091270007301800. Epub 2007 May 24. J Clin Pharmacol. 2007. PMID: 17525168 Clinical Trial.
-
Pharmacokinetic evaluation of fosaprepitant dimeglumine.Expert Opin Drug Metab Toxicol. 2010 Oct;6(10):1277-86. doi: 10.1517/17425255.2010.513970. Expert Opin Drug Metab Toxicol. 2010. PMID: 20795794 Free PMC article. Review.
-
Fosaprepitant dimeglumine (MK-0517 or L-785,298), an intravenous neurokinin-1 antagonist for the prevention of chemotherapy induced nausea and vomiting.Expert Opin Pharmacother. 2008 Dec;9(18):3261-70. doi: 10.1517/14656560802548463. Expert Opin Pharmacother. 2008. PMID: 19040346 Review.
Cited by
-
Crossover safety study of aprepitant: 2-min injection vs 30-min infusion in cancer patients receiving emetogenic chemotherapy.Onco Targets Ther. 2019 Apr 30;12:3277-3284. doi: 10.2147/OTT.S201609. eCollection 2019. Onco Targets Ther. 2019. PMID: 31118678 Free PMC article.
-
Challenges in the Development of Intravenous Neurokinin-1 Receptor Antagonists: Results of a Safety and Pharmacokinetics Dose-Finding, Phase 1 Study of Intravenous Fosnetupitant.Clin Pharmacol Drug Dev. 2022 Dec;11(12):1405-1418. doi: 10.1002/cpdd.1183. Epub 2022 Oct 20. Clin Pharmacol Drug Dev. 2022. PMID: 36263927 Free PMC article. Clinical Trial.
-
Safety Profile of HTX-019 Administered as an Intravenous Push in Cancer Patients: A Retrospective Review.Adv Ther. 2019 Mar;36(3):662-669. doi: 10.1007/s12325-019-0877-3. Epub 2019 Jan 31. Adv Ther. 2019. PMID: 30706408 Free PMC article.
-
Evolving role of neurokinin 1-receptor antagonists for chemotherapy-induced nausea and vomiting.Onco Targets Ther. 2018 Oct 4;11:6459-6478. doi: 10.2147/OTT.S158570. eCollection 2018. Onco Targets Ther. 2018. PMID: 30323622 Free PMC article. Review.
-
Phase IIIb Safety and Efficacy of Intravenous NEPA for Prevention of Chemotherapy-Induced Nausea and Vomiting (CINV) in Patients with Breast Cancer Receiving Initial and Repeat Cycles of Anthracycline and Cyclophosphamide (AC) Chemotherapy.Oncologist. 2020 Mar;25(3):e589-e597. doi: 10.1634/theoncologist.2019-0527. Epub 2019 Dec 8. Oncologist. 2020. PMID: 32162813 Free PMC article. Clinical Trial.
References
-
- Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24(27):4472–4478. - PubMed
-
- Haiderali A, Menditto L, Good M, Teitelbaum A, Wegner J. Impact on daily functioning and indirect/direct costs associated with chemotherapy-induced nausea and vomiting (CINV) in a U.S. population. Support Care Cancer. 2011;19(6):843–851. - PubMed
-
- Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997;15(1):103–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

