The effects of repeated Toll-like receptors 2 and 4 stimulation in COPD alveolar macrophages
- PMID: 29535517
- PMCID: PMC5841324
- DOI: 10.2147/COPD.S97071
The effects of repeated Toll-like receptors 2 and 4 stimulation in COPD alveolar macrophages
Abstract
Background: COPD is a progressive inflammatory airway disease characterized by increased numbers of alveolar macrophages in the lungs. Bacterial colonization of the lungs is a common feature in COPD and can promote inflammation through continual and repeated Toll-like receptor (TLR) stimulation. We have studied the response of COPD alveolar macrophages to repetitive stimulation with TLR2 and TLR4 ligands. We investigated the effect of sequential stimulation with different ligands to determine whether this results in tolerance or amplification of the immune response.
Methods: We stimulated alveolar macrophages from COPD patients (n=9) and smokers (n=8) with the TLR4 agonist lipopolysaccharide (LPS) or the TLR2 agonist Pam3CSK4 for 24 hours before restimulating again for 24 hours. Cytokine protein release and gene expression were investigated.
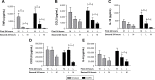
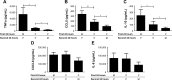
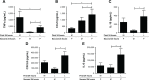
Results: Repetitive stimulation of COPD and smokers macrophages with LPS for both 24-hour periods caused a reduction in tumor necrosis factor α, CCL5, and IL-10 production compared to cells that were not exposed initially to LPS. IL-6 and CXCL8 production were not significantly altered following repetitive LPS stimulation. The same pattern was observed for repeated stimulation with Pam3CSK4. Using COPD macrophages, LPS followed by Pam3CSK4 stimulation increased the levels of all cytokines compared to media followed by Pam3CSK4.
Conclusion: TLR tolerance in COPD alveolar macrophages occurs after repetitive stimulation with the same TLR ligand, but this only occurs for selected cytokines. CXCL8 production is not reduced after repetitive TLR stimulation with the same ligand; this may be an important mechanism for the increased CXCL8 levels that have been observed in COPD. We showed that TLR4 stimulation followed by TLR2 stimulation does not cause tolerance, but enhances cytokine production. This may be a relevant mechanism by which bacteria cause excessive inflammation in COPD patients.
Keywords: COPD; alveolar macrophages; tolerance.
Conflict of interest statement
Disclosure SRL, SLR and MK have no conflicts of interest. KDS, SRH and EMH are employees of GSK. DS has received sponsorship to attend international meetings, honoraria for lecturing or attending advisory boards and research grants from various pharmaceutical companies including Apellis, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Genentech, Glaxo-SmithKline, Glenmark, Johnson and Johnson, Menarini, Mundipharma, Novartis, Peptinnovate Pfizer, Pulmatrix, Skypharma, Teva, Therevance and Verona. The authors report no other conflicts of interest in this work.
Figures
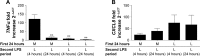

References
-
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. - PubMed
-
- Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. - PubMed
-
- Desai H, Eschberger K, Wrona C, et al. Bacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(3):303–309. - PubMed
-
- Taylor AE, Finney-Hayward TK, Quint JK, et al. Defective macrophage phagocytosis of bacteria in COPD. Eur Respir J. 2010;35(5):1039–1047. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

