Tetrathiomolybdate Treatment Leads to the Suppression of Inflammatory Responses through the TRAF6/NFκB Pathway in LPS-Stimulated BV-2 Microglia
- PMID: 29535623
- PMCID: PMC5835334
- DOI: 10.3389/fnagi.2018.00009
Tetrathiomolybdate Treatment Leads to the Suppression of Inflammatory Responses through the TRAF6/NFκB Pathway in LPS-Stimulated BV-2 Microglia
Abstract
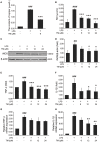
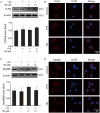
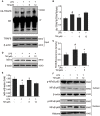
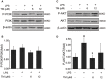
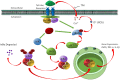
Although the positive relationship between copper and Alzheimer's disease (AD) was reported by a lot of epidemiological data, the mechanism is not completely known. Copper is a redox metal and serves as a mediator of inflammation. Because the homeostasis of copper is altered in Aβ precursor protein (APP) and presenilin 1 (PS1) transgenic (Tg) mice, the using of copper chelators is a potential therapeutic strategy for AD. Here we report that a copper chelator, tetrathiomolybdate (TM), is a potential therapeutic drug of AD. We investigated whether TM treatment led to a decrease of pro-inflammatory cytokines in vivo and in vitro, and found that TM treatment reduced the expression of iNOS and TNF-α in APP/PS1 Tg mice through up-regulating superoxide dismutase 1 (SOD1) activity. In vitro, once stimulated, microglia secretes a variety of proinflammatory cytokines, so we utilized LPS-stimulated BV-2 cells as the inflammatory cell model to detect the anti-inflammatory effects of TM. Our results indicated that TM-pretreatment suppressed the ubiquitination of TRAF6 and the activation of NFκB without affecting the expression of TLR4 and Myd88 in vitro. By detecting the activity of SOD1 and the production of reactive oxygen species (ROS), we found that the anti-inflammatory effects of TM could be attributed to its ability to reduce the amount of intracellular bioavailable copper, and the production of ROS which is an activator of the TRAF6 auto-ubiquitination. Hence, our results revealed that TM-treatment could reduce the production of inflammatory cytokines by the suppression of ROS/TRAF6/AKT/NFκB signaling pathway.
Keywords: ROS; copper; inflammation; microglia; tetrathiomolybdate.
Figures


Similar articles
-
Loganin prevents BV-2 microglia cells from Aβ1-42 -induced inflammation via regulating TLR4/TRAF6/NF-κB axis.Cell Biol Int. 2018 Dec;42(12):1632-1642. doi: 10.1002/cbin.11060. Epub 2018 Oct 31. Cell Biol Int. 2018. PMID: 30288860
-
Copper chelators promote nonamyloidogenic processing of AβPP via MT1/2 /CREB-dependent signaling pathways in AβPP/PS1 transgenic mice.J Pineal Res. 2018 Oct;65(3):e12502. doi: 10.1111/jpi.12502. Epub 2018 May 18. J Pineal Res. 2018. PMID: 29710396
-
Cytosolic phospholipase A2 plays a crucial role in ROS/NO signaling during microglial activation through the lipoxygenase pathway.J Neuroinflammation. 2015 Oct 31;12:199. doi: 10.1186/s12974-015-0419-0. J Neuroinflammation. 2015. PMID: 26520095 Free PMC article.
-
The blockage of the Nogo/NgR signal pathway in microglia alleviates the formation of Aβ plaques and tau phosphorylation in APP/PS1 transgenic mice.J Neuroinflammation. 2016 Mar 3;13(1):56. doi: 10.1186/s12974-016-0522-x. J Neuroinflammation. 2016. PMID: 26939570 Free PMC article.
-
Metal and inflammatory targets for Alzheimer's disease.Curr Drug Targets. 2004 Aug;5(6):535-51. doi: 10.2174/1389450043345272. Curr Drug Targets. 2004. PMID: 15270200 Review.
Cited by
-
Copper accumulation and the effect of chelation treatment on cerebral amyloid angiopathy compared to parenchymal amyloid plaques.Metallomics. 2020 Apr 1;12(4):539-546. doi: 10.1039/c9mt00306a. Epub 2020 Feb 27. Metallomics. 2020. PMID: 32104807 Free PMC article.
-
Neuroprotective effects of ammonium tetrathiomolybdate, a slow-release sulfide donor, in a rodent model of regional stroke.Intensive Care Med Exp. 2020 Apr 9;8(1):13. doi: 10.1186/s40635-020-00300-8. Intensive Care Med Exp. 2020. PMID: 32274608 Free PMC article.
-
Targeting PI3K/Akt signal transduction for cancer therapy.Signal Transduct Target Ther. 2021 Dec 16;6(1):425. doi: 10.1038/s41392-021-00828-5. Signal Transduct Target Ther. 2021. PMID: 34916492 Free PMC article. Review.
-
Copper and cuproptosis: new therapeutic approaches for Alzheimer's disease.Front Aging Neurosci. 2023 Dec 19;15:1300405. doi: 10.3389/fnagi.2023.1300405. eCollection 2023. Front Aging Neurosci. 2023. PMID: 38178962 Free PMC article. Review.
-
METH-Induced Neurotoxicity Is Alleviated by Lactulose Pretreatment Through Suppressing Oxidative Stress and Neuroinflammation in Rat Striatum.Front Neurosci. 2018 Nov 2;12:802. doi: 10.3389/fnins.2018.00802. eCollection 2018. Front Neurosci. 2018. PMID: 30450033 Free PMC article.
References
-
- Ardestani P. M., Evans A. K., Yi B., Nguyen T., Coutellier L., Shamloo M. (2017). Modulation of neuroinflammation and pathology in the 5XFAD mouse model of Alzheimer's disease using a biased and selective beta-1 adrenergic receptor partial agonist. Neuropharmacology 116, 371–386. 10.1016/j.neuropharm.2017.01.010 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

