Downregulation of TLR4 by miR-181a Provides Negative Feedback Regulation to Lipopolysaccharide-Induced Inflammation
- PMID: 29535629
- PMCID: PMC5834510
- DOI: 10.3389/fphar.2018.00142
Downregulation of TLR4 by miR-181a Provides Negative Feedback Regulation to Lipopolysaccharide-Induced Inflammation
Abstract
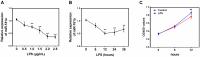
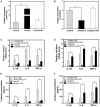
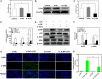
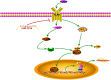
Acute lung injury (ALI) is a progressive clinical disease with a high mortality rate, and characterized by an excessive uncontrolled inflammatory response. MicroRNAs (miRNAs) play a critical role in various human inflammatory diseases, and have been recognized as important regulators of inflammation. However, the regulatory mechanisms mediated by miRNAs involved in Lipopolysaccharide (LPS)-induced inflammation in ALI remain hazy. In this study, we found that miR-181a expression in the lung tissues of ALI mice and LPS-stimulated RAW 264.7 macrophages is dramatically reduced. We also show that over-expression of miR-181a significantly decreased the production of inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, whereas inhibition of miR-181a reversed this decrease. Moreover, miR-181a inhibits NF-κB activation and accumulation of reactive oxygen species (ROS) by targeting TLR4 expression. We further verify that miR-181a suppresses TLR4 expression by binding directly to the 3'-UTR of TLR4. Therefore, we provide the first evidence for the negative regulation of miR-181a in LPS-induced inflammation via the suppression of ROS generation and TLR4-NF-κB pathway.
Keywords: LPS; NF-κB; ROS; acute lung injury; miR-181a.
Figures




Similar articles
-
MicroRNA-106a Provides Negative Feedback Regulation in Lipopolysaccharide-Induced Inflammation by targeting TLR4.Int J Biol Sci. 2019 Aug 22;15(11):2308-2319. doi: 10.7150/ijbs.33432. eCollection 2019. Int J Biol Sci. 2019. PMID: 31595149 Free PMC article.
-
MicroRNA-182 supplies negative feedback regulation to ameliorate lipopolysaccharide-induced ALI in mice by targeting TLR4.J Cell Physiol. 2020 Sep;235(9):5925-5937. doi: 10.1002/jcp.29504. Epub 2020 Jan 31. J Cell Physiol. 2020. PMID: 32003008
-
miR-27a protects against acute lung injury in LPS-treated mice by inhibiting NF-κB-mediated inflammatory response.Int J Clin Exp Pathol. 2018 Jun 1;11(6):2980-2989. eCollection 2018. Int J Clin Exp Pathol. 2018. PMID: 31938423 Free PMC article.
-
Over-expressed microRNA-181a reduces glomerular sclerosis and renal tubular epithelial injury in rats with chronic kidney disease via down-regulation of the TLR/NF-κB pathway by binding to CRY1.Mol Med. 2018 Sep 18;24(1):49. doi: 10.1186/s10020-018-0045-2. Mol Med. 2018. PMID: 30241461 Free PMC article.
-
Overexpression of miR-145-5p alleviated LPS-induced acute lung injury.J Biol Regul Homeost Agents. 2019 Jul-Aug;33(4):1063-1072. J Biol Regul Homeost Agents. 2019. PMID: 31353880
Cited by
-
Advancements in understanding the role of microRnas in regulating macrophage polarization during acute lung injury.Cell Cycle. 2023 Jul-Aug;22(14-16):1694-1712. doi: 10.1080/15384101.2023.2230018. Epub 2023 Jul 6. Cell Cycle. 2023. PMID: 37415386 Free PMC article. Review.
-
Environmental enrichment affects immunity and reduces disease severity in pigs after co-infection, with stronger effects when applied from birth than from weaning.Front Vet Sci. 2024 Dec 9;11:1511209. doi: 10.3389/fvets.2024.1511209. eCollection 2024. Front Vet Sci. 2024. PMID: 39720408 Free PMC article.
-
miRNAs in Pulmonary Hypertension: Mechanistic Insights and Therapeutic Potential.Biomedicines. 2025 Aug 5;13(8):1910. doi: 10.3390/biomedicines13081910. Biomedicines. 2025. PMID: 40868164 Free PMC article. Review.
-
Enforced expression of miR-92b blunts E. coli lipopolysaccharide-mediated inflammatory injury by activating the PI3K/AKT/β-catenin pathway via targeting PTEN.Int J Biol Sci. 2021 Mar 25;17(5):1289-1301. doi: 10.7150/ijbs.56933. eCollection 2021. Int J Biol Sci. 2021. PMID: 33867846 Free PMC article.
-
Mechanistic role of long non-coding RNAs in the pathogenesis of metabolic dysfunction-associated steatotic liver disease and fibrosis.eGastroenterology. 2024 Nov;2(4):e100115. doi: 10.1136/egastro-2024-100115. Epub 2024 Nov 18. eGastroenterology. 2024. PMID: 39872125 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

