Short-Term Sustained Hypoxia Elevates Basal and Hypoxia-Induced Ventilation but Not the Carotid Body Chemoreceptor Activity in Rats
- PMID: 29535636
- PMCID: PMC5835044
- DOI: 10.3389/fphys.2018.00134
Short-Term Sustained Hypoxia Elevates Basal and Hypoxia-Induced Ventilation but Not the Carotid Body Chemoreceptor Activity in Rats
Abstract
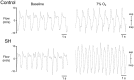
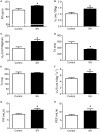
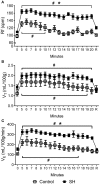
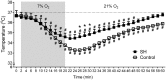
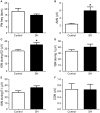
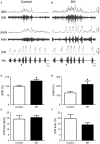
Exposure to chronic sustained hypoxia (SH), as experienced in high altitudes, elicits an increase in ventilation, named ventilatory acclimatization to hypoxia (VAH). We previously showed that rats exposed to short-term (24 h) SH exhibit enhanced abdominal expiratory motor activity at rest, accompanied by augmented baseline sympathetic vasoconstrictor activity. In the present study, we investigated whether the respiratory and sympathetic changes elicited by short-term SH are accompanied by carotid body chemoreceptor sensitization. Juvenile male Holtzman rats (60-80 g) were exposed to SH (10% O2 for 24 h) or normoxia (control) to examine basal and hypoxic-induced ventilatory parameters in unanesthetized conditions, as well as the sensory response of carotid body chemoreceptors in artificially perfused in situ preparations. Under resting conditions (normoxia/normocapnia), SH rats (n = 12) exhibited higher baseline respiratory frequency, tidal volume, and minute ventilation compared to controls (n = 11, P < 0.05). SH group also showed greater hypoxia ventilatory response than control group (P < 0.05). The in situ preparations of SH rats (n = 8) exhibited augmented baseline expiratory and sympathetic activities under normocapnia, with additional bursts in abdominal and thoracic sympathetic nerves during late expiratory phase that were not seen in controls (n = 8, P < 0.05). Interestingly, basal and potassium cyanide-induced afferent activity of carotid sinus nerve (CSN) was similar between SH and control rats. Our findings indicate that the maintenance of elevated resting ventilation, baseline sympathetic overactivity, and enhanced ventilatory responses to hypoxia in rats exposed to 24 h of SH are not dependent on increased basal and sensorial activity of carotid body chemoreceptors.
Keywords: active expiration; carotid body; chemoreceptor; hypoxic ventilatory response; sympathetic activity; ventilatory acclimatization.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

