The Role of Sirt6 in Obesity and Diabetes
- PMID: 29535637
- PMCID: PMC5835030
- DOI: 10.3389/fphys.2018.00135
The Role of Sirt6 in Obesity and Diabetes
Abstract
Sirt6 is one of the sirtuin family members, a kind of NAD+-dependent histone deacetylase and ADP-ribose transferase enzyme. It has an important role in physiological and pathological processes, regulating aging, cancer, obesity, insulin resistance, inflammation, and energy metabolism. Recent studies have suggested that reduced Sirt6 action is related to obesity and diabetes. Aging and overnutrition, two major risk factors for obesity and diabetes, lead to decreased Sirt6 level and function, which results in abnormal glucose and lipid metabolism. Whole-body ablation of Sirt6 in mice results in severe hypoglycemia. Sirt6 deficiency leads to liver steatosis and promotes diet-induced obesity and insulin resistance. Sirt6 has a protective effect on obesity and diabetes. This review surveys evidence for an emerging role of Sirt6 as a regulator of metabolism in mammals and summarizes its major functions in obesity and diabetes.
Keywords: LiPo; Sirt6; diabetes mellitus; gluconeogenesis; obesity; type 2.
Figures
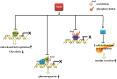
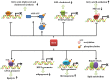
References
-
- Barbatelli G., Murano I., Madsen L., Hao Q., Jimenez M., Kristiansen K., et al. . (2010). The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 298, E1244–E1253. 10.1152/ajpendo.00600.2009 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

