Repeated Exposure to the "Spice" Cannabinoid JWH-018 Induces Tolerance and Enhances Responsiveness to 5-HT1A Receptor Stimulation in Male Rats
- PMID: 29535650
- PMCID: PMC5835089
- DOI: 10.3389/fpsyt.2018.00055
Repeated Exposure to the "Spice" Cannabinoid JWH-018 Induces Tolerance and Enhances Responsiveness to 5-HT1A Receptor Stimulation in Male Rats
Abstract
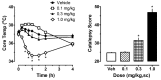
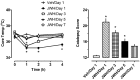
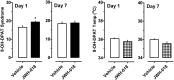
Naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) is a synthetic compound found in psychoactive "spice" products that activates cannabinoid receptors. Preclinical evidence suggests that exposure to synthetic cannabinoids increases 5-HT2A/2C receptor function in the brain, an effect which might contribute to psychotic symptoms. Here, we hypothesized that repeated exposures to JWH-018 would enhance behavioral responsiveness to the 5-HT2A/2C receptor agonist DOI. Male Sprague-Dawley rats fitted with subcutaneously (sc) temperature transponders received daily injections of JWH-018 (1.0 mg/kg, sc) or its vehicle for seven consecutive days. Body temperature and catalepsy scores were determined at 1, 2, and 4 h post-injection each day. At 1 and 7 days after the final repeated treatment, rats received a challenge injection of either DOI (0.1 mg/kg, sc) or the 5-HT1A receptor agonist 8-OH-DPAT (0.3 mg/kg, sc), then temperature and behavioral responses were assessed. Behaviors induced by DOI included wet dog shakes and back muscle contractions (i.e., skin jerks), while behaviors induced by 8-OH-DPAT included ambulation, forepaw treading, and flat body posture. On the first day of repeated treatment, JWH-018 produced robust hypothermia and catalepsy which lasted up to 4 h, and these effects were significantly blunted by day 7 of treatment. Repeated exposure to JWH-018 did not affect behaviors induced by DOI, but behavioral and hypothermic responses induced by 8-OH-DPAT were significantly augmented 1 day after cessation of JWH-018 treatment. Collectively, our findings show that repeated treatment with JWH-018 produces tolerance to its hypothermic and cataleptic effects, which is accompanied by transient enhancement of 5-HT1A receptor sensitivity in vivo.
Keywords: JWH-018; receptor; serotonin; spice; synthetic cannabinoid.
Figures



Similar articles
-
Pharmacodynamic Effects, Pharmacokinetics, and Metabolism of the Synthetic Cannabinoid AM-2201 in Male Rats.J Pharmacol Exp Ther. 2018 Dec;367(3):543-550. doi: 10.1124/jpet.118.250530. Epub 2018 Sep 28. J Pharmacol Exp Ther. 2018. PMID: 30266766 Free PMC article.
-
Repeated treatment with 8-OH-DPAT induces tolerance to its ability to produce the 5-HT1A behavioural syndrome, but not to its ability to attenuate haloperidol-induced catalepsy.Behav Pharmacol. 2000 Jun;11(3-4):299-305. doi: 10.1097/00008877-200006000-00013. Behav Pharmacol. 2000. PMID: 11103884
-
The 5-HT1A receptor full agonist, 8-OH-DPAT inhibits ACTH-induced 5-HT2A receptor hyperfunction in rats: involvement of 5-HT1A receptors in the DOI-induced wet-dog shakes in ACTH-treated rats.Biol Pharm Bull. 2007 Jan;30(1):117-20. doi: 10.1248/bpb.30.117. Biol Pharm Bull. 2007. PMID: 17202670
-
Repeated administration of phytocannabinoid Δ(9)-THC or synthetic cannabinoids JWH-018 and JWH-073 induces tolerance to hypothermia but not locomotor suppression in mice, and reduces CB1 receptor expression and function in a brain region-specific manner.Pharmacol Res. 2015 Dec;102:22-32. doi: 10.1016/j.phrs.2015.09.006. Epub 2015 Sep 8. Pharmacol Res. 2015. PMID: 26361728 Free PMC article.
-
The relation of central 5-HT1A and 5-HT2 receptors: low dose agonist-induced selective tolerance in the rat.Pharmacol Biochem Behav. 1991 Jun;39(2):407-13. doi: 10.1016/0091-3057(91)90199-c. Pharmacol Biochem Behav. 1991. PMID: 1835098
Cited by
-
Molecular Mechanisms of Action of Novel Psychoactive Substances (NPS). A New Threat for Young Drug Users with Forensic-Toxicological Implications.Life (Basel). 2021 May 14;11(5):440. doi: 10.3390/life11050440. Life (Basel). 2021. PMID: 34068903 Free PMC article. Review.
-
Behavioral Effects of Developmental Exposure to JWH-018 in Wild-Type and Disrupted in Schizophrenia 1 (disc1) Mutant Zebrafish.Biomolecules. 2021 Feb 19;11(2):319. doi: 10.3390/biom11020319. Biomolecules. 2021. PMID: 33669793 Free PMC article.
-
Pharmacokinetics and pharmacodynamics of the synthetic cannabinoid, 5F-MDMB-PICA, in male rats.Neuropharmacology. 2021 Nov 1;199:108800. doi: 10.1016/j.neuropharm.2021.108800. Epub 2021 Sep 20. Neuropharmacology. 2021. PMID: 34547333 Free PMC article.
-
Effects of the synthetic cannabinoid receptor agonist JWH-018 on abuse-related effects of opioids in rhesus monkeys.Drug Alcohol Depend. 2019 Sep 1;202:33-38. doi: 10.1016/j.drugalcdep.2019.04.024. Epub 2019 Jun 29. Drug Alcohol Depend. 2019. PMID: 31295696 Free PMC article.
-
Pharmacodynamic Effects, Pharmacokinetics, and Metabolism of the Synthetic Cannabinoid AM-2201 in Male Rats.J Pharmacol Exp Ther. 2018 Dec;367(3):543-550. doi: 10.1124/jpet.118.250530. Epub 2018 Sep 28. J Pharmacol Exp Ther. 2018. PMID: 30266766 Free PMC article.
References
-
- U.S. Drug Enforcement Administration, Office of Diversion Control. National Forensic Laboratory Information System: Year 2013 Annual Report. Springfield, VA: U.S. Drug Enforcement Administration; (2014).
-
- U.S. Drug Enforcement Administration, Diversion Control Division. National Forensic Laboratory Information System: Year 2015 Annual Report. Springfield, VA: U.S. Drug Enforcement Administration; (2016).
-
- Wiley JL, Compton DR, Dai D, Lainton JA, Phillips M, Huffman JW, et al. Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J Pharmacol Exp Ther (1998) 285:995–1004. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

