Lipoteichoic Acid Inhibits Staphylococcus aureus Biofilm Formation
- PMID: 29535693
- PMCID: PMC5835072
- DOI: 10.3389/fmicb.2018.00327
Lipoteichoic Acid Inhibits Staphylococcus aureus Biofilm Formation
Abstract
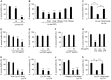
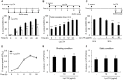
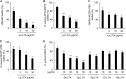
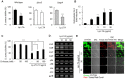
A biofilm is an aggregate of microorganisms in which cells adhere to biological or non-biological surfaces and is responsible for various infectious diseases. Infections caused by Staphylococcus aureus, including pneumonia, endocarditis, and osteomyelitis, are often associated with colonization and biofilm formation. Although lipoteichoic acid (LTA) is involved in biofilm formation, the specific role of LTA is not clearly understood. In this study, we demonstrated that LTA released from Lactobacillus plantarum could inhibit S. aureus biofilm formation and aggregation without affecting the growth of S. aureus in various in vitro and in vivo models. L. plantarum LTA (Lp.LTA) also inhibited biofilm formation of S. aureus clinical isolates, including a methicillin-resistant strain. Remarkably, Lp.LTA not only interfered with S. aureus biofilm formation, but it also disrupted a pre-formed biofilm. Mechanism studies demonstrated that Lp.LTA inhibited expression of the ica-operon, which is responsible for the production of poly-N-acetylglucosamine, a key molecule required for S. aureus biofilm development. Lp.LTA increased the release of autoinducer-2 from S. aureus, which contributed to the inhibition of S. aureus biofilm formation. Moreover, Lp.LTA treatment enhanced susceptibility of the biofilm to various antibiotics and to macrophages. Interestingly, Lp.LTA without D-alanine moieties was not able to inhibit biofilm formation by S. aureus. In conclusion, the present study suggests that LTA can inhibit S. aureus biofilm formation, and therefore could be applied for preventing and/or treating infectious diseases caused by S. aureus biofilms.
Keywords: Lactobacillus plantarum; Staphylococcus aureus; biofilm formation; infectious diseases; lipoteichoic acid.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

