Dectin-1 Positive Dendritic Cells Expand after Infection with Leishmania major Parasites and Represent Promising Targets for Vaccine Development
- PMID: 29535708
- PMCID: PMC5834765
- DOI: 10.3389/fimmu.2018.00263
Dectin-1 Positive Dendritic Cells Expand after Infection with Leishmania major Parasites and Represent Promising Targets for Vaccine Development
Abstract
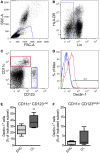
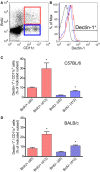
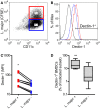
Resistant mouse strains mount a protective T cell-mediated immune response upon infection with Leishmania (L.) parasites. Healing correlates with a T helper (Th) cell-type 1 response characterized by a pronounced IFN-γ production, while susceptibility is associated with an IL-4-dependent Th2-type response. It has been shown that dermal dendritic cells are crucial for inducing protective Th1-mediated immunity. Additionally, there is growing evidence that C-type lectin receptor (CLR)-mediated signaling is involved in directing adaptive immunity against pathogens. However, little is known about the function of the CLR Dectin-1 in modulating Th1- or Th2-type immune responses by DC subsets in leishmaniasis. We characterized the expression of Dectin-1 on CD11c+ DCs in peripheral blood, at the site of infection, and skin-draining lymph nodes of L. major-infected C57BL/6 and BALB/c mice and in peripheral blood of patients suffering from cutaneous leishmaniasis (CL). Both mouse strains responded with an expansion of Dectin-1+ DCs within the analyzed tissues. In accordance with the experimental model, Dectin-1+ DCs expanded as well in the peripheral blood of CL patients. To study the role of Dectin-1+ DCs in adaptive immunity against L. major, we analyzed the T cell stimulating potential of bone marrow-derived dendritic cells (BMDCs) in the presence of the Dectin-1 agonist Curdlan. These experiments revealed that Curdlan induces the maturation of BMDCs and the expansion of Leishmania-specific CD4+ T cells. Based on these findings, we evaluated the impact of Curdlan/Dectin-1 interactions in experimental leishmaniasis and were able to demonstrate that the presence of Curdlan at the site of infection modulates the course of disease in BALB/c mice: wild-type BALB/c mice treated intradermally with Curdlan developed a protective immune response against L. major whereas Dectin-1-/- BALB/c mice still developed the fatal course of disease after Curdlan treatment. Furthermore, the vaccination of BALB/c mice with a combination of soluble L. major antigens and Curdlan was able to provide a partial protection from severe leishmaniasis. These findings indicate that the ligation of Dectin-1 on DCs acts as an important checkpoint in adaptive immunity against L. major and should therefore be considered in future whole-organism vaccination strategies.
Keywords: Curdlan; Dectin-1; T helper 1 and T helper 2 cells; adaptive immunity; cutaneous leishmaniasis; dendritic cells; β-glucan.
Figures






Similar articles
-
Interleukin-4 Responsive Dendritic Cells Are Dispensable to Host Resistance Against Leishmania mexicana Infection.Front Immunol. 2022 Jan 28;12:759021. doi: 10.3389/fimmu.2021.759021. eCollection 2021. Front Immunol. 2022. PMID: 35154068 Free PMC article.
-
T cell-derived IL-10 determines leishmaniasis disease outcome and is suppressed by a dendritic cell based vaccine.PLoS Pathog. 2013;9(6):e1003476. doi: 10.1371/journal.ppat.1003476. Epub 2013 Jun 27. PLoS Pathog. 2013. PMID: 23825956 Free PMC article.
-
Leishmania major-infected murine langerhans cell-like dendritic cells from susceptible mice release IL-12 after infection and vaccinate against experimental cutaneous Leishmaniasis.Eur J Immunol. 2000 Dec;30(12):3498-506. doi: 10.1002/1521-4141(2000012)30:12<3498::AID-IMMU3498>3.0.CO;2-6. Eur J Immunol. 2000. PMID: 11093169
-
Persistent parasites and immunologic memory in cutaneous leishmaniasis: implications for vaccine designs and vaccination strategies.Immunol Res. 2008;41(2):123-36. doi: 10.1007/s12026-008-8016-2. Immunol Res. 2008. PMID: 18389179 Review.
-
Anti-leishmania effector functions of CD4+ Th1 cells and early events instructing Th2 cell development and susceptibility to Leishmania major in BALB/c mice.Adv Exp Med Biol. 1998;452:53-60. doi: 10.1007/978-1-4615-5355-7_7. Adv Exp Med Biol. 1998. PMID: 9889959 Review.
Cited by
-
Inhibition of Dectin-1 on Dendritic Cells Prevents Maturation and Prolongs Murine Islet Allograft Survival.J Inflamm Res. 2021 Jan 12;14:63-73. doi: 10.2147/JIR.S287453. eCollection 2021. J Inflamm Res. 2021. PMID: 33469336 Free PMC article.
-
The Effect of Curcumin-Loaded Glucan Nanoparticles on Immune Cells: Size as a Critical Quality Attribute.Pharmaceutics. 2023 Feb 13;15(2):623. doi: 10.3390/pharmaceutics15020623. Pharmaceutics. 2023. PMID: 36839945 Free PMC article.
-
Molecular Aspects of Dendritic Cell Activation in Leishmaniasis: An Immunobiological View.Front Immunol. 2019 Feb 22;10:227. doi: 10.3389/fimmu.2019.00227. eCollection 2019. Front Immunol. 2019. PMID: 30873156 Free PMC article. Review.
-
C-Type Lectins in Veterinary Species: Recent Advancements and Applications.Int J Mol Sci. 2020 Jul 20;21(14):5122. doi: 10.3390/ijms21145122. Int J Mol Sci. 2020. PMID: 32698416 Free PMC article. Review.
-
Distinct functions of CAR-T cells possessing a dectin-1 intracellular signaling domain.Gene Ther. 2023 May;30(5):411-420. doi: 10.1038/s41434-021-00257-7. Epub 2021 May 6. Gene Ther. 2023. PMID: 33953316
References
-
- Solbach W, Bogdan C, Moll H, Lohoff M, Rollinghoff M. [Parasitic evasion mechanisms: Leishmania as an example]. Immun Infekt (1989) 17(4):129–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

