Vaccines for Leprosy and Tuberculosis: Opportunities for Shared Research, Development, and Application
- PMID: 29535713
- PMCID: PMC5834475
- DOI: 10.3389/fimmu.2018.00308
Vaccines for Leprosy and Tuberculosis: Opportunities for Shared Research, Development, and Application
Abstract
Tuberculosis (TB) and leprosy still represent significant public health challenges, especially in low- and lower middle-income countries. Both poverty-related mycobacterial diseases require better tools to improve disease control. For leprosy, there has been an increased emphasis on developing tools for improved detection of infection and early diagnosis of disease. For TB, there has been a similar emphasis on such diagnostic tests, while increased research efforts have also focused on the development of new vaccines. Bacille Calmette-Guérin (BCG), the only available TB vaccine, provides insufficient and inconsistent protection to pulmonary TB in adults. The impact of BCG on leprosy, however, is significant, and the introduction of new TB vaccines that might replace BCG could, therefore, have serious impact also on leprosy. Given the similarities in antigenic makeup between the pathogens Mycobacterium tuberculosis (Mtb) and M. leprae, it is well possible, however, that new TB vaccines could cross-protect against leprosy. New TB subunit vaccines currently evaluated in human phase I and II studies indeed often contain antigens with homologs in M. leprae. In this review, we discuss pre-clinical studies and clinical trials of subunit or whole mycobacterial vaccines for TB and leprosy and reflect on the development of vaccines that could provide protection against both diseases. Furthermore, we provide the first preclinical evidence of such cross-protection by Mtb antigen 85B (Ag85B)-early secretory antigenic target (ESAT6) fusion recombinant proteins in in vivo mouse models of Mtb and M. leprae infection. We propose that preclinical integration and harmonization of TB and leprosy research should be considered and included in global strategies with respect to cross-protective vaccine research and development.
Keywords: Mycobacterium leprae; Mycobacterium tuberculosis; antigen 85B; early secretory antigenic target; hybrid recombinant protein; leprosy; tuberculosis; vaccines.
Figures


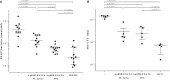
Similar articles
-
rBCG30-induced immunity and cross-protection against Mycobacterium leprae challenge are enhanced by boosting with the Mycobacterium tuberculosis 30-kilodalton antigen 85B.Infect Immun. 2014 Sep;82(9):3900-9. doi: 10.1128/IAI.01499-13. Epub 2014 Jul 7. Infect Immun. 2014. PMID: 25001602 Free PMC article.
-
Protection against Mycobacterium leprae infection by the ID83/GLA-SE and ID93/GLA-SE vaccines developed for tuberculosis.Infect Immun. 2014 Sep;82(9):3979-85. doi: 10.1128/IAI.02145-14. Epub 2014 Jul 14. Infect Immun. 2014. PMID: 25024362 Free PMC article.
-
DNA-Launched Alphavirus Replicons Encoding a Fusion of Mycobacterial Antigens Acr and Ag85B Are Immunogenic and Protective in a Murine Model of TB Infection.PLoS One. 2015 Aug 28;10(8):e0136635. doi: 10.1371/journal.pone.0136635. eCollection 2015. PLoS One. 2015. PMID: 26317509 Free PMC article.
-
Leprosy and tuberculosis vaccine design.Trop Med Parasitol. 1989 Sep;40(3):251-7. Trop Med Parasitol. 1989. PMID: 2482532 Review.
-
Genome wide approaches discover novel Mycobacterium tuberculosis antigens as correlates of infection, disease, immunity and targets for vaccination.Semin Immunol. 2018 Oct;39:88-101. doi: 10.1016/j.smim.2018.07.001. Epub 2018 Jul 7. Semin Immunol. 2018. PMID: 30327124 Review.
Cited by
-
A Bibliometric Analysis of Leprosy during 2000-2021 from Web of Science Database.Int J Environ Res Public Health. 2022 Jul 6;19(14):8234. doi: 10.3390/ijerph19148234. Int J Environ Res Public Health. 2022. PMID: 35886085 Free PMC article.
-
Mycobacterium leprae: A historical study on the origins of leprosy and its social stigma.Infez Med. 2021 Dec 10;29(4):623-632. doi: 10.53854/liim-2904-18. eCollection 2021. Infez Med. 2021. PMID: 35146374 Free PMC article.
-
Effectiveness of population-wide screening and mass drug administration for leprosy control in Kiribati: the COMBINE protocol.BMJ Open. 2023 Jun 29;13(6):e065369. doi: 10.1136/bmjopen-2022-065369. BMJ Open. 2023. PMID: 37385746 Free PMC article.
-
Unlocking Opportunities for Mycobacterium leprae and Mycobacterium ulcerans.ACS Infect Dis. 2024 Feb 9;10(2):251-269. doi: 10.1021/acsinfecdis.3c00371. Epub 2024 Jan 31. ACS Infect Dis. 2024. PMID: 38295025 Free PMC article. Review.
-
The impact of single-cell genomics on the field of mycobacterial infection.Front Microbiol. 2022 Sep 30;13:989464. doi: 10.3389/fmicb.2022.989464. eCollection 2022. Front Microbiol. 2022. PMID: 36246265 Free PMC article. Review.
References
-
- van Hooij A, Geluk A. Immunodiagnostics for leprosy. In: Scollard DM, Gillis TP, editors. International Textbook of Leprosy. (2016). Available from: www.internationaltextbookofleprosy.org
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

