Amino Acids As Mediators of Metabolic Cross Talk between Host and Pathogen
- PMID: 29535717
- PMCID: PMC5835074
- DOI: 10.3389/fimmu.2018.00319
Amino Acids As Mediators of Metabolic Cross Talk between Host and Pathogen
Abstract
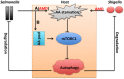
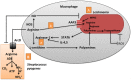
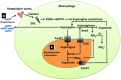
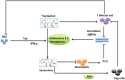
The interaction between host and pathogen decidedly shapes the outcome of an infection, thus understanding this interaction is critical to the treatment of a pathogen-induced infection. Although research in this area of cell biology has yielded surprising findings regarding interactions between host and pathogen, understanding of the metabolic cross talk between host and pathogen is limited. At the site of infection, host and pathogen share similar or identical nutritional substrates and generate common metabolic products, thus metabolic cross talk between host and pathogen could profoundly affect the pathogenesis of an infection. In this review, we present results of a recent discovery of a metabolic interaction between host and pathogen from an amino acid (AA) metabolism-centric point of view. The host depends on AA metabolism to support defensive responses against pathogens, while the pathogens modulate AA metabolism for its own advantage. Some AA, such as arginine, asparagine, and tryptophan, are central points of competition between the host and pathogen. Thus, a better understanding of AA-mediated metabolic cross talk between host and pathogen will provide insight into fruitful therapeutic approaches to manipulate and prevent progression of an infection.
Keywords: amino acids; arginine; asparagine; infection; metabolism.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

