The MEK1/2-ERK Pathway Inhibits Type I IFN Production in Plasmacytoid Dendritic Cells
- PMID: 29535732
- PMCID: PMC5835309
- DOI: 10.3389/fimmu.2018.00364
The MEK1/2-ERK Pathway Inhibits Type I IFN Production in Plasmacytoid Dendritic Cells
Abstract
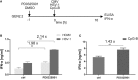
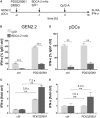
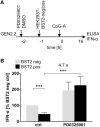
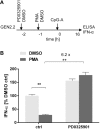
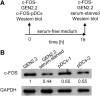
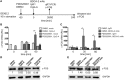
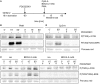
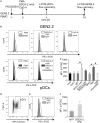
Recent studies have reported that the crosslinking of regulatory receptors (RRs), such as blood dendritic cell antigen 2 (BDCA-2) (CD303) or ILT7 (CD85g), of plasmacytoid dendritic cells (pDCs) efficiently suppresses the production of type I interferons (IFN-I, α/β/ω) and other cytokines in response to toll-like receptor 7 and 9 (TLR7/9) ligands. The exact mechanism of how this B cell receptor (BCR)-like signaling blocks TLR7/9-mediated IFN-I production is unknown. Here, we stimulated BCR-like signaling by ligation of RRs with BDCA-2 and ILT7 mAbs, hepatitis C virus particles, or BST2 expressing cells. We compared BCR-like signaling in proliferating pDC cell line GEN2.2 and in primary pDCs from healthy donors, and addressed the question of whether pharmacological targeting of BCR-like signaling can antagonize RR-induced pDC inhibition. To this end, we tested the TLR9-mediated production of IFN-I and proinflammatory cytokines in pDCs exposed to a panel of inhibitors of signaling molecules involved in BCR-like, MAPK, NF-ĸB, and calcium signaling pathways. We found that MEK1/2 inhibitors, PD0325901 and U0126 potentiated TLR9-mediated production of IFN-I in GEN2.2 cells. More importantly, MEK1/2 inhibitors significantly increased the TLR9-mediated IFN-I production blocked in both GEN2.2 cells and primary pDCs upon stimulation of BCR-like or phorbol 12-myristate 13-acetate-induced protein kinase C (PKC) signaling. Triggering of BCR-like and PKC signaling in pDCs resulted in an upregulation of the expression and phoshorylation of c-FOS, a downstream gene product of the MEK1/2-ERK pathway. We found that the total level of c-FOS was higher in proliferating GEN2.2 cells than in the resting primary pDCs. The PD0325901-facilitated restoration of the TLR9-mediated IFN-I production correlated with the abrogation of MEK1/2-ERK-c-FOS signaling. These results indicate that the MEK1/2-ERK pathway inhibits TLR9-mediated type I IFN production in pDCs and that pharmacological targeting of MEK1/2-ERK signaling could be a strategy to overcome immunotolerance of pDCs and re-establish their immunogenic activity.
Keywords: B cell-like receptor signaling; MEK1/2; blood dendritic cell antigen 2; c-FOS; plasmacytoid dendritic cells; regulatory receptors; toll-like receptors 7 and 9 (TLR7/9); type I interferon.
Figures



Similar articles
-
Dual Role of the Tyrosine Kinase Syk in Regulation of Toll-Like Receptor Signaling in Plasmacytoid Dendritic Cells.PLoS One. 2016 Jun 3;11(6):e0156063. doi: 10.1371/journal.pone.0156063. eCollection 2016. PLoS One. 2016. PMID: 27258042 Free PMC article.
-
Bruton's tyrosine kinase regulates TLR9 but not TLR7 signaling in human plasmacytoid dendritic cells.Eur J Immunol. 2014 Apr;44(4):1130-6. doi: 10.1002/eji.201344030. Epub 2014 Jan 20. Eur J Immunol. 2014. PMID: 24375473
-
HBsAg inhibits TLR9-mediated activation and IFN-alpha production in plasmacytoid dendritic cells.Mol Immunol. 2009 Aug;46(13):2640-6. doi: 10.1016/j.molimm.2009.04.031. Epub 2009 Jun 5. Mol Immunol. 2009. PMID: 19501403
-
Regulation of TLR7/9 signaling in plasmacytoid dendritic cells.Protein Cell. 2013 Jan;4(1):40-52. doi: 10.1007/s13238-012-2104-8. Epub 2012 Nov 7. Protein Cell. 2013. PMID: 23132256 Free PMC article. Review.
-
Signaling and ligand interaction of ILT7: receptor-mediated regulatory mechanisms for plasmacytoid dendritic cells.Immunol Rev. 2010 Mar;234(1):163-76. doi: 10.1111/j.0105-2896.2009.00867.x. Immunol Rev. 2010. PMID: 20193018 Free PMC article. Review.
Cited by
-
Plasmacytoid Dendritic Cell Impairment in Metastatic Melanoma by Lactic Acidosis.Cancers (Basel). 2020 Jul 28;12(8):2085. doi: 10.3390/cancers12082085. Cancers (Basel). 2020. PMID: 32731406 Free PMC article.
-
MEK1/2 Inhibitors Unlock the Constrained Interferon Response in Macrophages Through IRF1 Signaling.Front Immunol. 2019 Aug 27;10:2020. doi: 10.3389/fimmu.2019.02020. eCollection 2019. Front Immunol. 2019. PMID: 31507609 Free PMC article.
-
Identification of hepatitis B virus and liver cancer bridge molecules based on functional module network.World J Gastroenterol. 2019 Sep 7;25(33):4921-4932. doi: 10.3748/wjg.v25.i33.4921. World J Gastroenterol. 2019. PMID: 31543683 Free PMC article.
-
Tumor cell intrinsic RON signaling suppresses innate immune responses in breast cancer through inhibition of IRAK4 signaling.Cancer Lett. 2021 Apr 10;503:75-90. doi: 10.1016/j.canlet.2021.01.019. Epub 2021 Jan 27. Cancer Lett. 2021. PMID: 33508385 Free PMC article.
-
Beyond Tethering the Viral Particles: Immunomodulatory Functions of Tetherin (BST-2).DNA Cell Biol. 2019 Nov;38(11):1170-1177. doi: 10.1089/dna.2019.4777. Epub 2019 Sep 9. DNA Cell Biol. 2019. PMID: 31502877 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

