Neuropilin 1 Mediates Keratinocyte Growth Factor Signaling in Adipose-Derived Stem Cells: Potential Involvement in Adipogenesis
- PMID: 29535768
- PMCID: PMC5845512
- DOI: 10.1155/2018/1075156
Neuropilin 1 Mediates Keratinocyte Growth Factor Signaling in Adipose-Derived Stem Cells: Potential Involvement in Adipogenesis
Abstract
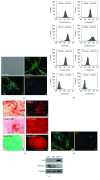
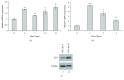
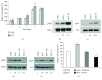
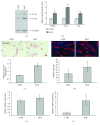
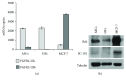
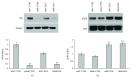
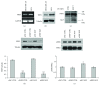
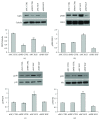
Adipogenesis is regulated by a complex network of molecules, including fibroblast growth factors. Keratinocyte growth factor (KGF) has been previously reported to promote proliferation on rat preadipocytes, although the expression of its specific receptor, FGFR2-IIIb/KGFR, is not actually detected in mesenchymal cells. Here, we demonstrate that human adipose-derived stem cells (ASCs) show increased expression of KGF during adipogenic differentiation, especially in the early steps. Moreover, KGF is able to induce transient activation of ERK and p38 MAPK pathways in these cells. Furthermore, KGF promotes ASC differentiation and supports the activation of differentiation pathways, such as those of PI3K/Akt and the retinoblastoma protein (Rb). Notably, we observed only a low amount of FGFR2-IIIb in ASCs, which seems not to be responsible for KGF activity. Here, we demonstrate for the first time that Neuropilin 1 (NRP1), a transmembrane glycoprotein expressed in ASCs acting as a coreceptor for some growth factors, is responsible for KGF-dependent pathway activation in these cells. Our study contributes to clarify the molecular bases of human adipogenesis, demonstrating a role of KGF in the early steps of this process, and points out a role of NRP1 as a previously unknown mediator of KGF action in ASCs.
Figures

Similar articles
-
Keratinocyte growth factor promotes preadipocyte proliferation via an autocrine mechanism.J Cell Biochem. 2010 Mar 1;109(4):737-46. doi: 10.1002/jcb.22452. J Cell Biochem. 2010. PMID: 20069574
-
Keratinocyte growth factor stimulates growth of MIA PaCa-2 cells through extracellular signal-regulated kinase phosphorylation.Oncol Lett. 2012 Feb;3(2):307-310. doi: 10.3892/ol.2011.466. Epub 2011 Oct 31. Oncol Lett. 2012. PMID: 22740901 Free PMC article.
-
Enhancement of keratinocyte growth factor potential in inducing adipose-derived stem cells differentiation into keratinocytes by collagen-targeting.J Cell Mol Med. 2022 Dec;26(23):5929-5942. doi: 10.1111/jcmm.17619. Epub 2022 Nov 21. J Cell Mol Med. 2022. PMID: 36412036 Free PMC article.
-
Activation of mitogen-activated protein kinase pathway by keratinocyte growth factor or fibroblast growth factor-10 promotes cell proliferation in human endometrial carcinoma cells.J Clin Endocrinol Metab. 2003 Feb;88(2):773-80. doi: 10.1210/jc.2002-021062. J Clin Endocrinol Metab. 2003. PMID: 12574212
-
Keratinocyte growth factor as a cytokine that mediates mesenchymal-epithelial interaction.EXS. 1995;74:191-214. doi: 10.1007/978-3-0348-9070-0_10. EXS. 1995. PMID: 8527895 Review.
Cited by
-
Adipose tissue-derived stem cells: a comparative review on isolation, culture, and differentiation methods.Cell Tissue Bank. 2022 Mar;23(1):1-16. doi: 10.1007/s10561-021-09905-z. Epub 2021 Feb 22. Cell Tissue Bank. 2022. PMID: 33616792 Review.
-
3D culturing as a promising strategy to enhance the angiogenic potential of adipose stem cell-derived secretome: insights into the role of miR-145-5p/ANGPT2 axis.Stem Cell Res Ther. 2025 Mar 28;16(1):153. doi: 10.1186/s13287-025-04277-7. Stem Cell Res Ther. 2025. PMID: 40155988 Free PMC article.
-
Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application.Front Cell Dev Biol. 2020 Apr 17;8:236. doi: 10.3389/fcell.2020.00236. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32363193 Free PMC article. Review.
-
Deciphering the Transcriptional Metabolic Profile of Adipose-Derived Stem Cells During Osteogenic Differentiation and Epigenetic Drug Treatment.Cells. 2025 Jan 17;14(2):135. doi: 10.3390/cells14020135. Cells. 2025. PMID: 39851564 Free PMC article.
-
Neuropilin 1 (NRP1) Positively Regulates Adipogenic Differentiation in C3H10T1/2 Cells.Int J Mol Sci. 2023 Apr 17;24(8):7394. doi: 10.3390/ijms24087394. Int J Mol Sci. 2023. PMID: 37108554 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

