Superior efficacy of the antifungal agent ciclopirox olamine over gemcitabine in pancreatic cancer models
- PMID: 29535812
- PMCID: PMC5828195
- DOI: 10.18632/oncotarget.23164
Superior efficacy of the antifungal agent ciclopirox olamine over gemcitabine in pancreatic cancer models
Abstract
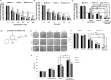
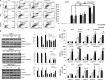
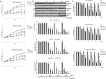
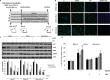
Ciclopirox olamine (CPX) is an antifungal agent that has recently demonstrated promising anti-neoplastic activity against hematologic and solid tumors. Here, we evaluated CPX compared with gemcitabine alone as well as their combination in human pancreatic cancer cell lines; BxPC-3, Panc-1, and MIA PaCa-2 and in humanized xenograft mouse models. We also examined the preclinical pharmacodynamic activity of CPX. CPX caused a pronounced decrease in cell proliferation and clonogenic growth potential. These inhibitory effects were accompanied by induction of reactive oxygen species (ROS), which were strongly associated with reduced Bcl-xL and survivin levels and activation of a panel of caspases, especially caspase-3, and finally resulted in apoptotic death. CPX-induced apoptosis was associated with reduced pEGFR (Y1068) and pAkt (Ser473) protein levels. Additionally, decreased proliferation was observed in CPX-treated xenografts tumors, demonstrating unique tumor regression and a profound survival benefit. Finally, we showed that CPX significantly abrogated gemcitabine-induced ROS levels in pancreatic tissues. These pre-clinical results have verified the superior antitumor efficacy of CPX over gemcitabine alone, while their combination is even more effective, providing the rationale for further clinical testing of CPX plus gemcitabine in pancreatic cancer patients.
Keywords: ciclopirox olamine; gemcitabine; human pancreatic tumor xenograft models; pancreatic cancer; pharmacodynamic activity.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no potential conflicts of interest
Figures


Similar articles
-
Reposition of the Fungicide Ciclopirox for Cancer Treatment.Recent Pat Anticancer Drug Discov. 2021;16(2):122-135. doi: 10.2174/1574892816666210211090845. Recent Pat Anticancer Drug Discov. 2021. PMID: 33573561 Free PMC article. Review.
-
Ciclopirox Olamine Exerts Tumor-Suppressor Effects via Topoisomerase II Alpha in Lung Adenocarcinoma.Front Oncol. 2022 Feb 18;12:791916. doi: 10.3389/fonc.2022.791916. eCollection 2022. Front Oncol. 2022. PMID: 35251970 Free PMC article.
-
Reactive oxygen and nitrogen species are crucial for the antifungal activity of amorolfine and ciclopirox olamine against the dermatophyte Trichophyton interdigitale.Med Mycol. 2022 Aug 18;60(8):myac058. doi: 10.1093/mmy/myac058. Med Mycol. 2022. PMID: 35896502
-
CPX Targeting DJ-1 Triggers ROS-induced Cell Death and Protective Autophagy in Colorectal Cancer.Theranostics. 2019 Jul 28;9(19):5577-5594. doi: 10.7150/thno.34663. eCollection 2019. Theranostics. 2019. PMID: 31534504 Free PMC article.
-
Antileukemia Effect of Ciclopirox Olamine Is Mediated by Downregulation of Intracellular Ferritin and Inhibition β-Catenin-c-Myc Signaling Pathway in Glucocorticoid Resistant T-ALL Cell Lines.PLoS One. 2016 Aug 23;11(8):e0161509. doi: 10.1371/journal.pone.0161509. eCollection 2016. PLoS One. 2016. PMID: 27551974 Free PMC article.
Cited by
-
Ciclopirox olamine induces ferritinophagy and reduces cyst burden in polycystic kidney disease.JCI Insight. 2021 Mar 30;6(8):e141299. doi: 10.1172/jci.insight.141299. JCI Insight. 2021. PMID: 33784251 Free PMC article.
-
Efficacy and non-toxicity of ciclopirox olamine-loaded liposomes against Cryptococcus neoformans clinical isolates.Braz J Microbiol. 2023 Sep;54(3):1513-1521. doi: 10.1007/s42770-023-01071-6. Epub 2023 Aug 4. Braz J Microbiol. 2023. PMID: 37540461 Free PMC article.
-
Reposition of the Fungicide Ciclopirox for Cancer Treatment.Recent Pat Anticancer Drug Discov. 2021;16(2):122-135. doi: 10.2174/1574892816666210211090845. Recent Pat Anticancer Drug Discov. 2021. PMID: 33573561 Free PMC article. Review.
-
Fosciclopirox suppresses growth of high-grade urothelial cancer by targeting the γ-secretase complex.Cell Death Dis. 2021 May 31;12(6):562. doi: 10.1038/s41419-021-03836-z. Cell Death Dis. 2021. PMID: 34059639 Free PMC article.
-
Sugar and iron: Toward understanding the antibacterial effect of ciclopirox in Escherichia coli.PLoS One. 2019 Jan 11;14(1):e0210547. doi: 10.1371/journal.pone.0210547. eCollection 2019. PLoS One. 2019. PMID: 30633761 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA: A Cancer Journal for Clinicians. 2017;67:7–30. - PubMed
-
- Adiseshaiah PP, Crist RM, Hook SS, McNeil SE. Nanomedicine strategies to overcome the pathophysiological barriers of pancreatic cancer. Nature Reviews Clinical Oncology. 2016;13:750–65. - PubMed
-
- Groot VP, van Santvoort HC, Rombouts SJE, Hagendoorn J, Borel Rinkes IHM, van Vulpen M, Herman JM, Wolfgang CL, Besselink MG, Molenaar IQ. Systematic review on the treatment of isolated local recurrence of pancreatic cancer after surgery; re-resection, chemoradiotherapy and SBRT. HPB (Oxford) 2017;19:83–92. - PubMed
-
- Davidson NE, Armstrong SA, Coussens LM, Cruz-Correa MR, DeBerardinis RJ, Doroshow JH, Foti M, Hwu P, Kensler TW, Morrow M, Mulligan CG, Pao W, Platz EA, et al. AACR Cancer Progress Report 2016. Clinical Cancer Research. American Association for Cancer Research. 2016;22:S1–S137. - PubMed
-
- Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. The Lancet. 2016. pp. 73–85. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

