An Exploratory Trial of Transdermal Nicotine for Aggression and Irritability in Adults with Autism Spectrum Disorder
- PMID: 29536216
- PMCID: PMC6394231
- DOI: 10.1007/s10803-018-3536-7
An Exploratory Trial of Transdermal Nicotine for Aggression and Irritability in Adults with Autism Spectrum Disorder
Abstract
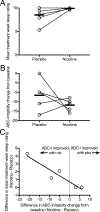
Nicotinic acetylcholine receptors (nAChRs), particularly the α7 nAChR, are implicated in the pathophysiology of both autism spectrum disorder (ASD) and aggressive behavior. We explored the feasibility, tolerability, and preliminary efficacy of targeting nAChRs using transdermal nicotine to reduce aggressive symptoms in adults with ASD. Eight subjects were randomized in a double-blind crossover trial of 7 mg transdermal nicotine or placebo, each for 1 week. All participants tolerated nicotine treatment well. Five subjects contributed data to the primary outcome, Aberrant Behavior Checklist-Irritability (ABC-I) subscale change from baseline, which was improved by nicotine compared to placebo. Sleep ratings were also improved by nicotine and correlated with ABC-I improvement. These findings support further investigation of nAChR agonists for aggression and sleep in ASD.
Keywords: Adult; Aggression; Autism spectrum disorder; Irritability; Nicotine; Nicotinic acetylcholine receptor; Sleep.
Conflict of interest statement
Conflict of interest
The authors declare no conflicts of interest.
Figures
References
-
- Aman MG, Singh NN, Stewart AW, Field CJ (1985). The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. Am J Ment Defic 89, 485–91 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH077681/MH/NIMH NIH HHS/United States
- T32MH14276/MH/NIMH NIH HHS/United States
- T32 MH019961/MH/NIMH NIH HHS/United States
- Child Study Center Associates/Child Study Center, Yale School of Medicine/International
- Pilot Award for General Psychiatry Residents/American Academy of Child and Adolescent Psychiatry/International
- 9699/AS/Autism Speaks/United States
- R01DA14241/DA/NIDA NIH HHS/United States
- R25MH071584/MH/NIMH NIH HHS/United States
- R01MH077681/MH/NIMH NIH HHS/United States
- R01 DA014241/DA/NIDA NIH HHS/United States
- R25 MH071584/MH/NIMH NIH HHS/United States
- T32 MH014276/MH/NIMH NIH HHS/United States
- T32MH019961/MH/NIMH NIH HHS/United States
- R37 DA014241/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

