Functional Status and Well-Being in People with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Compared with People with Multiple Sclerosis and Healthy Controls
- PMID: 29536371
- PMCID: PMC6249197
- DOI: 10.1007/s41669-018-0071-6
Functional Status and Well-Being in People with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Compared with People with Multiple Sclerosis and Healthy Controls
Abstract
Background: People with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) continue to struggle to have their condition recognised as disabling in the face of public and professional prejudice and discrimination.
Objective: The aim of this study was to compare the functional status and well-being of people with well-characterised ME/CFS with people with multiple sclerosis (PWMS), as well as healthy controls (HCs).
Methods: In this cross-sectional study, we used data collected as part of the UK ME/CFS Biobank to compare actual participant scores from the Medical Outcomes Survey Short Form-36 v2™ (SF-36v2™) between groups, as a proxy for impact of disability, and from a bespoke questionnaire seeking data on employment and income.
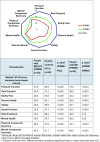
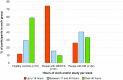
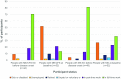
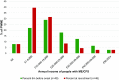
Results: People with ME/CFS scored significantly lower than PWMS or HCs in almost all SF-36v2™ areas. Prominent were lower scores for people with ME/CFS in the Physical Component Summary and Role Physical and Social Function domains, while the smallest differences were seen in the Mental Health domain. Responses to the bespoke questionnaire indicated that people with ME/CFS in this study work fewer hours and have lower incomes compared with people in the other two groups.
Conclusions: Using SF-36v2™ scores as a proxy, people with ME/CFS were measurably more disabled than PWMS or HCs in this study population. Furthermore, employment and income data are consistent with loss of functional status. These findings should encourage the health community to recognise the disabling effects of ME/CFS, to advocate for the needs of people with ME/CFS, and to investigate strategies to address the cost of the disease to both individuals and society.
Conflict of interest statement
Conflicts of Interest
Caroline Kingdon, Erinna Bowman, Hayley Curran, Luis Nacul, and Eliana Lacerda declare that they have no conflicts of interest.
Human Participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Ethical Approval
The UK ME/CFS Biobank received ethical approval from the London School of Hygiene & Tropical Medicine (LSHTM) Ethics Committee (ref. 6123), the National Research Ethics Service (NRES) London-Bloomsbury Research Ethics Committee (REC; ref. 11/LO/1760, IRAS ID: 77765), and the NHS Research Governance and Developments Offices (R&D), which oversee the recruitment of research participants from government health services.
Informed Consent
Written informed consent was obtained from all individual participants included in the study. Participants were encouraged to ask questions and were free to withdraw at any time.
Figures
References
-
- Olive M. The politics of disablement: a sociological approach critical texts in social work and the welfare state. Basingstoke: Macmillan and St Martin’s Press; 1990.
-
- ME Association. ESA—changes to the Working Capability Assessment descriptors. 2011. http://www.meassociation.org.uk/2011/02/esa-–-changes-to-the-working-cap.... Accessed 28 Feb 2018.
-
- Strassheim V, et al. What is known about severe and very severe chronic fatigue syndrome? A scoping review. Fatigue Biomed Health Behav. 2017;5(3):167–183. doi: 10.1080/21641846.2017.1333185. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

