Reproducible network and regional topographies of abnormal glucose metabolism associated with progressive supranuclear palsy: Multivariate and univariate analyses in American and Chinese patient cohorts
- PMID: 29536636
- PMCID: PMC6033655
- DOI: 10.1002/hbm.24044
Reproducible network and regional topographies of abnormal glucose metabolism associated with progressive supranuclear palsy: Multivariate and univariate analyses in American and Chinese patient cohorts
Abstract
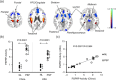
Progressive supranuclear palsy (PSP) is a rare movement disorder and often difficult to distinguish clinically from Parkinson's disease (PD) and multiple system atrophy (MSA) in early phases. In this study, we report reproducible disease-related topographies of brain network and regional glucose metabolism associated with PSP in clinically-confirmed independent cohorts of PSP, MSA, and PD patients and healthy controls in the USA and China. Using 18 F-FDG PET images from PSP and healthy subjects, we applied spatial covariance analysis with bootstrapping to identify a PSP-related pattern (PSPRP) and estimate its reliability, and evaluated the ability of network scores for differential diagnosis. We also detected regional metabolic differences using statistical parametric mapping analysis. We produced a highly reliable PSPRP characterized by relative metabolic decreases in the middle prefrontal cortex/cingulate, ventrolateral prefrontal cortex, striatum, thalamus and midbrain, covarying with relative metabolic increases in the hippocampus, insula and parieto-temporal regions. PSPRP network scores correlated positively with PSP duration and accurately discriminated between healthy, PSP, MSA and PD groups in two separate cohorts of parkinsonian patients at both early and advanced stages. Moreover, PSP patients shared many overlapping areas with abnormal metabolism in the same cortical and subcortical regions as in the PSPRP. With rigorous cross-validation, this study demonstrated highly comparable and reproducible PSP-related metabolic topographies at network and regional levels across different patient populations and PET scanners. Metabolic brain network activity may serve as a reliable and objective marker of PSP, although cross-validation applying recent diagnostic criteria and classification is warranted.
Keywords: PET; differential diagnosis; disease-related brain network markers; movement disorders; multivariate and univariate brain mapping methods; parkinsonism.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
D. E. serves on the scientific advisory board for and has received honoraria from the Michael J. Fox Foundation for Parkinson's Research; serves on the editorial board of Annals of Neurology and Journal of Nuclear Medicine and as Associate Editor for The Journal of Neuroscience; and is listed as coinventor of patents re: Markers for use in screening patients for nervous system dysfunction and a method and apparatus for using same, without financial gain; and has received research support from the NIH (NINDS, NIDCD, NIAID), the Dana Foundation, the Bachmann‐Strauss Dystonia and Parkinson Foundation, and CHDI Foundation. Y. M. has received research funding from the NIH. All other authors have no disclosures/conflicts to report.
Figures






References
-
- Agosta, F. , Kostic, V. S. , Galantucci, S. , Mesaros, S. , Svetel, M. , Pagani, E. , … Filippi, M. (2010). The in vivo distribution of brain tissue loss in Richardson's syndrome and PSP‐parkinsonism: A VBM‐DARTEL study. The European Journal of Neuroscience, 32(4), 640–647. 10.1111/j.1460-9568.2010.07304.x - DOI - PubMed
-
- Albin, R. L. , Young, A. B. , & Penney, J. B. (1989). The functional anatomy of basal ganglia disorders. Trends in Neurosciences, 12(10), 366–375. - PubMed
-
- Amtage, F. , Maurer, C. , Hellwig, S. , Tuscher, O. , Kreft, A. , Weiller, C. , … Meyer, P. T. (2014). Functional correlates of vertical gaze palsy and other ocular motor deficits in PSP: An FDG‐PET study. Parkinsonism & Related Disorders, 20(8), 898–906. 10.1016/j.parkreldis.2014.05.013 - DOI - PubMed
-
- Anderson, T. J. , Jenkins, I. H. , Brooks, D. J. , Hawken, M. B. , Frackowiak, R. S. , & Kennard, C. (1994). Cortical control of saccades and fixation in man. A PET study. Brain, 117(5), 1073–1084. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

