Most bicarbonate secretion by Calu-3 cells is mediated by CFTR and independent of pendrin
- PMID: 29536650
- PMCID: PMC5849580
- DOI: 10.14814/phy2.13641
Most bicarbonate secretion by Calu-3 cells is mediated by CFTR and independent of pendrin
Abstract
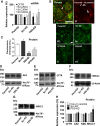
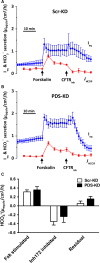
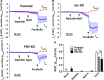
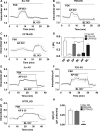
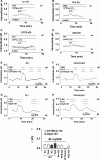
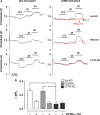
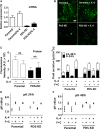
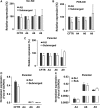
Bicarbonate plays an important role in airway host defense, however, its transport mechanisms remain uncertain. Here we examined the relative contributions of the anion channel CFTR (cystic fibrosis transmembrane conductance regulator, ABCC7) and the anion exchanger pendrin (SLC26A4) to HCO3- secretion by the human airway cell line Calu-3. Pendrin and CFTR were both detected in parental Calu-3 cells, although mRNA and protein expression appeared higher for CFTR than for pendrin. Targeting pendrin transcripts with lentiviral shRNA reduced pendrin detection by immunofluorescence staining but did not alter the rates of HCO3- or fluid secretion, HCO3- transport under pH-stat conditions, or net HCO3- flux across basolaterally permeabilized monolayers. Intracellular pH varied with step changes in apical Cl- and HCO3- concentrations in control and pendrin knockdown Calu-3 cells, but not in CFTR deficient cells. Exposure to the proinflammatory cytokine IL-4, which strongly upregulates pendrin expression in airway surface epithelia, had little effect on Calu-3 pendrin expression and did not alter fluid or HCO3- secretion. Similar results were obtained using air-liquid interface and submerged cultures, although CFTR and pendrin mRNA expression were both lower when cells were cultured under submerged conditions. While the conclusions cannot be extrapolated to other airway epithelia, the present results demonstrate that most HCO3- secretion by Calu-3 cells is mediated by CFTR.
Keywords: Airway epithelial cells; SLC26A4; cystic fibrosis; pendrin.
© 2018 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures

Similar articles
-
Pendrin Mediates Bicarbonate Secretion and Enhances Cystic Fibrosis Transmembrane Conductance Regulator Function in Airway Surface Epithelia.Am J Respir Cell Mol Biol. 2019 Jun;60(6):705-716. doi: 10.1165/rcmb.2018-0158OC. Am J Respir Cell Mol Biol. 2019. PMID: 30742493
-
Novel role for pendrin in orchestrating bicarbonate secretion in cystic fibrosis transmembrane conductance regulator (CFTR)-expressing airway serous cells.J Biol Chem. 2011 Nov 25;286(47):41069-82. doi: 10.1074/jbc.M111.266734. Epub 2011 Sep 13. J Biol Chem. 2011. PMID: 21914796 Free PMC article.
-
Protein phosphatase 1 coordinates CFTR-dependent airway epithelial HCO3- secretion by reciprocal regulation of apical and basolateral membrane Cl(-)-HCO3- exchangers.Br J Pharmacol. 2013 Apr;168(8):1946-60. doi: 10.1111/bph.12085. Br J Pharmacol. 2013. PMID: 23215877 Free PMC article.
-
Functional interplay between CFTR and pendrin: physiological and pathophysiological relevance.Front Biosci (Landmark Ed). 2022 Feb 21;27(2):75. doi: 10.31083/j.fbl2702075. Front Biosci (Landmark Ed). 2022. PMID: 35227018 Review.
-
Airway Surface Liquid pH Regulation in Airway Epithelium Current Understandings and Gaps in Knowledge.Int J Mol Sci. 2021 Mar 25;22(7):3384. doi: 10.3390/ijms22073384. Int J Mol Sci. 2021. PMID: 33806154 Free PMC article. Review.
Cited by
-
Airway surface liquid acidification initiates host defense abnormalities in Cystic Fibrosis.Sci Rep. 2019 Apr 24;9(1):6516. doi: 10.1038/s41598-019-42751-4. Sci Rep. 2019. PMID: 31019198 Free PMC article.
-
Case report: A case of SLC26A4 mutations causing pendred syndrome and non-cystic fibrosis bronchiectasis.Front Pediatr. 2023 Jan 9;10:1077878. doi: 10.3389/fped.2022.1077878. eCollection 2022. Front Pediatr. 2023. PMID: 36699303 Free PMC article.
-
Human Cellular Models for the Investigation of Lung Inflammation and Mucus Production in Cystic Fibrosis.Anal Cell Pathol (Amst). 2018 Nov 15;2018:3839803. doi: 10.1155/2018/3839803. eCollection 2018. Anal Cell Pathol (Amst). 2018. PMID: 30581723 Free PMC article. Review.
-
PHAIR: a biosensor for pH measurement in air-liquid interface cell culture.Sci Rep. 2021 Feb 10;11(1):3477. doi: 10.1038/s41598-021-83189-x. Sci Rep. 2021. PMID: 33568708 Free PMC article.
-
Effects of indacaterol on the LPS-evoked changes in fluid secretion rate and pH in swine tracheal membrane.Pflugers Arch. 2021 Jun;473(6):883-896. doi: 10.1007/s00424-021-02560-z. Epub 2021 May 24. Pflugers Arch. 2021. PMID: 34031755 Free PMC article.
References
-
- Alesutan, I. , Daryadel A., Mohebbi N., Pelzl L., Leibrock C., Voelkl J., et al. 2011. Impact of bicarbonate, ammonium chloride, and acetazolamide on hepatic and renal SLC26A4 expression. Cell. Physiol. Biochem. 28:553–558. - PubMed
-
- Bidart, J. M. , Lacroix L., Evain‐Brion D., Caillou B., Lazar V., Frydman R., et al. 2000. Expression of Na+/I‐ symporter and Pendred syndrome genes in trophoblast cells. J. Clin. Endocrinol. Metab. 85:4367–4372. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

