The lantibiotic gallidermin acts bactericidal against Staphylococcus epidermidis and Staphylococcus aureus and antagonizes the bacteria-induced proinflammatory responses in dermal fibroblasts
- PMID: 29536668
- PMCID: PMC6291784
- DOI: 10.1002/mbo3.606
The lantibiotic gallidermin acts bactericidal against Staphylococcus epidermidis and Staphylococcus aureus and antagonizes the bacteria-induced proinflammatory responses in dermal fibroblasts
Abstract
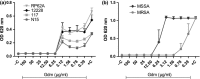
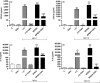
Antimicrobial resistance needs to be tackled from new angles, and antimicrobial peptides could be future candidates for combating bacterial infections. This study aims to investigate in vitro the bactericidal effects of the lantibiotic gallidermin on Staphylococcus epidermidis and Staphylococcus aureus, possible cytotoxic effects and its impact on host-microbe interactions. Minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) of gallidermin were determined, and cytotoxicity and proinflammatory effects of gallidermin on fibroblasts, red blood cells (RBCs) and in whole blood were investigated. Both MIC and MBC for all four tested strains of S. epidermidis was 6.25 μg/ml. Both MIC and MBC for methicillin-sensitive S. aureus was 12.5 μg/ml and for methicillin-resistant S. aureus (MRSA) 1.56 μg/ml. Gallidermin displayed no cytotoxic effects on fibroblasts, only a high dose of gallidermin induced low levels of CXCL8 and interleukin-6. Gallidermin hemolyzed less than 1% of human RBCs, and did not induce reactive oxygen species production or cell aggregation in whole blood. In cell culture, gallidermin inhibited the cytotoxic effects of the bacteria and totally suppressed the bacteria-induced release of CXCL8 and interleukin-6 from fibroblasts. We demonstrate that gallidermin, expressing low cell cytotoxicity, is a promising candidate for treating bacterial infections caused by S. epidermidis and S. aureus, especially MRSA.
Keywords: Streptococcus; antimicrobial resistance; bacteriocin; cytokines; fibroblasts; gallidermin.
© 2018 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Figures





Similar articles
-
Activity of gallidermin on Staphylococcus aureus and Staphylococcus epidermidis biofilms.Antimicrob Agents Chemother. 2012 Nov;56(11):5804-10. doi: 10.1128/AAC.01296-12. Epub 2012 Aug 27. Antimicrob Agents Chemother. 2012. PMID: 22926575 Free PMC article.
-
Antibacterial and anti-biofilm activities of thiazolidione derivatives against clinical staphylococcus strains.Emerg Microbes Infect. 2015 Jan;4(1):e1. doi: 10.1038/emi.2015.1. Epub 2015 Jan 7. Emerg Microbes Infect. 2015. PMID: 26038759 Free PMC article.
-
The two-component regulatory systems GraRS and SrrAB mediate Staphylococcus aureus susceptibility to Pep5 produced by clinical isolate of Staphylococcus epidermidis.Appl Environ Microbiol. 2024 Jul 24;90(7):e0030024. doi: 10.1128/aem.00300-24. Epub 2024 Jun 4. Appl Environ Microbiol. 2024. PMID: 38832774 Free PMC article.
-
Epidermin and gallidermin: Staphylococcal lantibiotics.Int J Med Microbiol. 2014 Jan;304(1):63-71. doi: 10.1016/j.ijmm.2013.08.012. Epub 2013 Sep 4. Int J Med Microbiol. 2014. PMID: 24119540 Review.
-
In-vitro profile of a new beta-lactam, ceftobiprole, with activity against methicillin-resistant Staphylococcus aureus.Clin Microbiol Infect. 2007 Jun;13 Suppl 2:17-24. doi: 10.1111/j.1469-0691.2007.01722.x. Clin Microbiol Infect. 2007. PMID: 17488372 Review.
Cited by
-
Staphylococcal-Produced Bacteriocins and Antimicrobial Peptides: Their Potential as Alternative Treatments for Staphylococcus aureus Infections.Antibiotics (Basel). 2020 Jan 21;9(2):40. doi: 10.3390/antibiotics9020040. Antibiotics (Basel). 2020. PMID: 31973108 Free PMC article. Review.
-
A Review: The Fate of Bacteriocins in the Human Gastro-Intestinal Tract: Do They Cross the Gut-Blood Barrier?Front Microbiol. 2018 Sep 28;9:2297. doi: 10.3389/fmicb.2018.02297. eCollection 2018. Front Microbiol. 2018. PMID: 30323796 Free PMC article. Review.
-
A Staphylococcus capitis strain with unusual bacteriocin production.Microb Biotechnol. 2023 Nov;16(11):2181-2193. doi: 10.1111/1751-7915.14356. Epub 2023 Oct 18. Microb Biotechnol. 2023. PMID: 37850940 Free PMC article.
-
Efficacy of Phage- and Bacteriocin-Based Therapies in Combatting Nosocomial MRSA Infections.Front Mol Biosci. 2021 Apr 29;8:654038. doi: 10.3389/fmolb.2021.654038. eCollection 2021. Front Mol Biosci. 2021. PMID: 33996906 Free PMC article. Review.
-
Application of bacteriocins in food preservation and infectious disease treatment for humans and livestock: a review.RSC Adv. 2020 Oct 23;10(64):38937-38964. doi: 10.1039/d0ra06161a. eCollection 2020 Oct 21. RSC Adv. 2020. PMID: 35518417 Free PMC article. Review.
References
-
- Breukink, E. , van Heusden, H. E. , Vollmerhaus, P. J. , Swiezewska, E. , Brunner, L. , Walker, S. , … de Kruijff, B. (2003). Lipid II is an intrinsic component of the pore induced by nisin in bacterial membranes. The Journal of Biological Chemistry, 278(22), 19898–19903. 10.1074/jbc.M301463200 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

