Introduction of pyrrolidineoxy or piperidineamino group at the 4-position of quinazoline leading to novel quinazoline-based phosphoinositide 3-kinase delta (PI3Kδ) inhibitors
- PMID: 29536777
- PMCID: PMC6009876
- DOI: 10.1080/14756366.2018.1444608
Introduction of pyrrolidineoxy or piperidineamino group at the 4-position of quinazoline leading to novel quinazoline-based phosphoinositide 3-kinase delta (PI3Kδ) inhibitors
Abstract
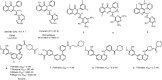
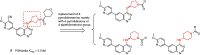
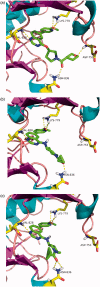
Phosphoinositide 3-kinase Delta (PI3Kδ) plays a key role in B-cell signal transduction and inhibition of PI3Kδ was confirmed to have clinical benefit in certain types of activation of B-cell malignancies. Herein, we reported a novel series of 4-pyrrolidineoxy or 4-piperidineamino substituted quinazolines, showing potent PI3Kδ inhibitory activities. Among these compounds, 12d, 14b and 14c demonstrated higher potency against PI3Kδ with the half maximal inhibitory concentration (IC50) values of 4.5, 3.0, and 3.9 nM, respectively, which were comparable to idelalisib (IC50 = 2.7 nM). The further PI3K isoforms selectivity evaluation showed that compounds 12d, 14b and 14c have excellent PI3Kδ selectivity over PI3Kα, PI3Kβ, and PI3Kγ. Moreover, compounds 12d, 14b and 14c also displayed different anti-proliferative profiles against a panel of four human B cell lines including Ramos, Raji, RPMI-8226, and SU-DHL-6. The molecular docking simulation indicated several key hydrogen bonding interactions were formed. This study suggests the introduction of pyrrolidineoxy or piperidineamino groups into the 4-position of quinazoline leads to new potent and selective PI3Kδ inhibitors.
Keywords: 4-piperidineamino; 4-pyrrolidineoxy; PI3Kδ inhibitors; anti-proliferation; selectivity.
Figures


References
-
- Ciraolo E, Morello F, Hirsch E.. Present and future of PI3K pathway inhibition in cancer: perspectives and limitations. Curr Med Chem 2011;18:2674–85. - PubMed
-
- Wei M, Wang X, Song Z, et al. . Targeting PI3Kδ: emerging therapy for chronic lymphocytic leukemia and beyond. Med Res Rev 2015;35:720–52. - PubMed
-
- Fruman DA, Rommel C.. PI3Kδ inhibitors in cancer: rationale and serendipity merge in the clinic. Cancer Discov 2011; 1:562–72. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
