Melting proteins confined in nanodroplets with 10.6 μm light provides clues about early steps of denaturation
- PMID: 29536995
- PMCID: PMC5871606
- DOI: 10.1039/c7cc09829d
Melting proteins confined in nanodroplets with 10.6 μm light provides clues about early steps of denaturation
Abstract
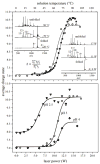
Ubiquitin confined within nanodroplets was irradiated with a variable-power CO2 laser. Mass spectrometry analysis shows evidence for a protein "melting"-like transition within droplets prior to solvent evaporation and ion formation. Ion mobility spectrometry reveals that structures associated with early steps of denaturation are trapped because of short droplet lifetimes.
Figures


Similar articles
-
Understanding the Thermal Denaturation of Myoglobin with IMS-MS: Evidence for Multiple Stable Structures and Trapped Pre-equilibrium States.J Am Soc Mass Spectrom. 2021 Jan 6;32(1):64-72. doi: 10.1021/jasms.0c00075. Epub 2020 Jul 7. J Am Soc Mass Spectrom. 2021. PMID: 32539412 Free PMC article.
-
On the structural denaturation of biological analytes in trapped ion mobility spectrometry - mass spectrometry.Analyst. 2016 Jun 7;141(12):3722-30. doi: 10.1039/c5an02399h. Analyst. 2016. PMID: 26998732
-
Melting Proteins: Evidence for Multiple Stable Structures upon Thermal Denaturation of Native Ubiquitin from Ion Mobility Spectrometry-Mass Spectrometry Measurements.J Am Chem Soc. 2017 May 10;139(18):6306-6309. doi: 10.1021/jacs.7b02774. Epub 2017 Apr 26. J Am Chem Soc. 2017. PMID: 28427262
-
Confinement Dynamics of Nanodroplets between Two Surfaces: Effects of Wettability and Electric Field.Chemphyschem. 2022 Dec 16;23(24):e202200184. doi: 10.1002/cphc.202200184. Epub 2022 Sep 12. Chemphyschem. 2022. PMID: 35986551
-
Charging and supercharging of proteins for mass spectrometry: recent insights into the mechanisms of electrospray ionization.Analyst. 2019 Nov 7;144(21):6157-6171. doi: 10.1039/c9an01201j. Epub 2019 Sep 27. Analyst. 2019. PMID: 31560020 Review.
Cited by
-
Substance P in the Gas Phase: Conformational Changes and Dissociations Induced by Collisional Activation in a Drift Tube.J Am Soc Mass Spectrom. 2019 Jun;30(6):932-945. doi: 10.1007/s13361-019-02160-3. Epub 2019 Apr 12. J Am Soc Mass Spectrom. 2019. PMID: 30980379 Free PMC article.
-
Variable-Temperature Native Mass Spectrometry for Studies of Protein Folding, Stabilities, Assembly, and Molecular Interactions.Annu Rev Biophys. 2022 May 9;51:63-77. doi: 10.1146/annurev-biophys-102221-101121. Epub 2021 Dec 21. Annu Rev Biophys. 2022. PMID: 34932911 Free PMC article. Review.
-
Understanding the Thermal Denaturation of Myoglobin with IMS-MS: Evidence for Multiple Stable Structures and Trapped Pre-equilibrium States.J Am Soc Mass Spectrom. 2021 Jan 6;32(1):64-72. doi: 10.1021/jasms.0c00075. Epub 2020 Jul 7. J Am Soc Mass Spectrom. 2021. PMID: 32539412 Free PMC article.
-
Time-resolved universal temperature measurements using NaYF4:Er3+,Yb3+ upconverting nanoparticles in an electrospray jet.Beilstein J Nanotechnol. 2018 Nov 21;9:2916-2924. doi: 10.3762/bjnano.9.270. eCollection 2018. Beilstein J Nanotechnol. 2018. PMID: 30546988 Free PMC article.
-
Dissociation of Macromolecules in Laser-Heated Droplets Monitored by CD-MS.Anal Chem. 2025 Jan 21;97(2):1419-1425. doi: 10.1021/acs.analchem.4c06038. Epub 2025 Jan 7. Anal Chem. 2025. PMID: 39772511
References
-
- Lee SW, Freivogel P, Schindler T, Beauchamp J. J Am Chem Soc. 1998;120:11758.
- Silveira JA, Fort KL, Pierson NA, Clemmer DE, Russell DH. J Am Chem Soc. 2013;135:19147. - PubMed
-
- Lumry R, Eyring H. J Phys Chem. 1954;58:110.
-
- Rodrigues RC, Ortiz C, Berenguer-Murcia A, Torres R, Fernandez-Lafuente R. Chem Soc Rev. 2013;42:6290. - PubMed
- Sanchez A, Cruz J, Rueda N, dos Santos JCS, Torres R, Ortiz C, Villalonga R, Fernández-Lafuente R. RSC Adv. 2016;6:27329.
-
- Lenkinski RE, Chen DM, Glickson JD, Goldstein G. Biochim Biophys Acta. 1977;494:126. - PubMed
- Cary PD, King DS, Crane-Robinson C, Bradbury WM, Rabbani A, Goodwin GH, Johns EW. Eur J Biochem. 1980;112:577. - PubMed
- Vijay-Kumar S, Bugg CE, Cook WJ. J Mol Biol. 1987;194:531. - PubMed
- Wintrode PL, Makhatadze GI, Privalov PL. Proteins: Struct, Funct, Genet. 1994;18:246. - PubMed
- Briggs MS, Roder H. Proc Natl Acad Sci U S A. 1992;89:2017. - PMC - PubMed
- Jackson SE. Org Biomol Chem. 2006;4:1845. - PubMed
- Kony DB, Hunenberger PH, van Gunsteren WF. Protein Sci. 2007;16:1101. - PMC - PubMed
-
- Wilkinson KD, Mayer AN. Arch Biochem Biophys. 1986;250:390. - PubMed
- Harding MM, Williams DH, Woolfson DN. Biochemistry. 1991;30:3120. - PubMed
- Pan Y, Briggs MS. Biochemistry. 1992;31:11405. - PubMed
- Stockman BJ, Euvrard A, Scahill TA. J Biomol NMR. 1993;3:285. - PubMed
- Cox JPL, Evans PA, Packman LC, Williams DH, Woolfson DN. J Mol Biol. 1993;234:483. - PubMed
- Brutscher B, Brüschweiler R, Ernst RR. Biochemistry. 1997;36:13043. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

