Single-inhaler triple therapy utilizing the once-daily combination of fluticasone furoate, umeclidinium and vilanterol in the management of COPD: the current evidence base and future prospects
- PMID: 29537340
- PMCID: PMC5941662
- DOI: 10.1177/1753466618760779
Single-inhaler triple therapy utilizing the once-daily combination of fluticasone furoate, umeclidinium and vilanterol in the management of COPD: the current evidence base and future prospects
Abstract
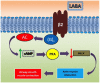
Maintenance pharmacological treatment for stable chronic obstructive pulmonary disease (COPD) is based on inhaled drugs, including long-acting muscarinic receptor antagonists (LAMA), long-acting β2-adrenoceptor agonists (LABA) and inhaled corticosteroids (ICS). Inhaled pharmacological treatment can improve patients' daily symptoms and reduce decline of pulmonary function and acute exacerbation rate. Treatment with all three inhaled drug classes is reserved for selected, more severe, patients with COPD when symptoms are not sufficiently controlled by dual LABA/LAMA therapy and exacerbations are frequent. This review focuses on the role of single-inhaler triple therapy with once-daily fluticasone furoate/umeclidinium/vilanterol fixed-dose combination, which is in phase III clinical development for maintenance treatment of severe-to-very severe COPD. In this review, we summarize evidence providing the rationale for its use in COPD and discuss the gaps to be filled in this pharmacotherapeutic area.
Keywords: COPD; fluticasone; pharmacotherapy; single-inhaler; triple therapy; umeclidinium; vilanterol.
Conflict of interest statement
Figures

References
-
- Miravitlles M, Soriano JB, García-Río F, et al. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax 2009; 64: 863–868. - PubMed
-
- López-Campos JL, Ruiz-Ramos M, Soriano JB. Mortality trends in chronic obstructive pulmonary disease in Europe, 1994–2010: a joinpoint regression analysis. Lancet Respir Med 2014; 2: 54–62. - PubMed
-
- Hutchinson A, Brand C, Irving L, et al. Acute care costs of patients admitted for management of chronic obstructive pulmonary disease exacerbations: contribution of disease severity, infection and chronic heart failure. Intern Med J 2010; 40: 364–371. - PubMed
-
- Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359: 1543–1554. - PubMed
-
- Vestbo J Anderson JA Brook RD et al.;. SUMMIT Investigators. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet 2016; 387: 1817–1826. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

