Chelating Rotaxane Ligands as Fluorescent Sensors for Metal Ions
- PMID: 29537728
- PMCID: PMC5947674
- DOI: 10.1002/anie.201712931
Chelating Rotaxane Ligands as Fluorescent Sensors for Metal Ions
Abstract
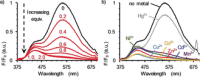
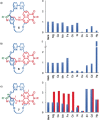
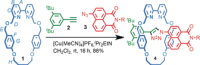
Although metal-ion-binding interlocked molecules have been under intense investigation for over three decades, their application as scaffolds for the development of sensors for metal ions remains underexplored. In this work, we demonstrate the potential of simple rotaxanes as metal-ion-responsive ligand scaffolds through the development of a proof-of-concept selective sensor for Zn2+ .
Keywords: fluorescent probes; rotaxanes; sensors; supramolecular chemistry; zinc.
© 2018 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

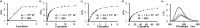
References
-
- Reviews:
-
- Domaille D. W., Que E. L., Chang C. J., Nat. Chem. Biol. 2008, 4, 168; - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

