Impairment of Meristem Proliferation in Plants Lacking the Mitochondrial Protease AtFTSH4
- PMID: 29538317
- PMCID: PMC5877714
- DOI: 10.3390/ijms19030853
Impairment of Meristem Proliferation in Plants Lacking the Mitochondrial Protease AtFTSH4
Abstract
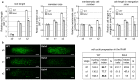
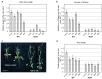
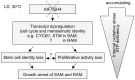
Shoot and root apical meristems (SAM and RAM, respectively) are crucial to provide cells for growth and organogenesis and therefore need to be maintained throughout the life of a plant. However, plants lacking the mitochondrial protease AtFTSH4 exhibit an intriguing phenotype of precocious cessation of growth at both the shoot and root apices when grown at elevated temperatures. This is due to the accumulation of internal oxidative stress and progressive mitochondria dysfunction. To explore the impacts of the internal oxidative stress on SAM and RAM functioning, we study the expression of selected meristem-specific (STM, CLV3, WOX5) and cell cycle-related (e.g., CYCB1, CYCD3;1) genes at the level of the promoter activity and/or transcript abundance in wild-type and loss-of-function ftsh4-1 mutant plants grown at 30 °C. In addition, we monitor cell cycle progression directly in apical meristems and analyze the responsiveness of SAM and RAM to plant hormones. We show that growth arrest in the ftsh4-1 mutant is caused by cell cycle dysregulation in addition to the loss of stem cell identity. Both the SAM and RAM gradually lose their proliferative activity, but with different timing relative to CYCB1 transcriptional activity (a marker of G2-M transition), which cannot be compensated by exogenous hormones.
Keywords: Arabidopsis; cell divisions; mitochondria; oxidative stress; root apical meristem; shoot apical meristem.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Evert R.F., Eichhorn S.E., editors. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development. 3rd ed. John Wiley & Sons; New York, NY, USA: 2007.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

